What You Need To Know About Stem Cells
You may have heard about stem cells in the news or maybe it was mentioned by a friend. After searching for more about it online, you may have come across websites or scientific journals that discussed the topic in a very complicated way.
You are not alone. While there are a lot of people who are interested in learning about stem cell-based treatments, there’s also a lot of clutter online and even misleading information about what it is and how therapies are administered. Contrary to popular belief, it does not take one stem cell injection or therapy session to treat your disease. It often takes a series of treatments and a personalized medical plan.
So, if you are considering stem cell treatment for your condition or you simply want to learn more about it, you have come to the right place. Here, we break down all the important things you need to know about stem cells in the simplest way possible, so you can make your important decision with a clear mind.
We’ve listed down and answered all the frequently asked questions about stem cells, including:
- How to choose your stem cell clinic or product?
- What is the potential of stem cells?
- What are stem cells?
- Why are they important?
- Where do they come from?
- How does stem cell therapy work?
- How much do stem cell therapies cost?
- What diseases do stem cell therapies treat?
Learn about all these and more in our comprehensive guide to stem cells and the diseases stem cell therapy can help treat.
How to Choose a Stem Cell Clinic?
How to Ensure Safety of a Treatment?
The most important point when choosing a stem cell treatment should always be your safety. Make sure that the stem cell clinic is officially controlled and works under GFP/GMP. In Germany, the regulatory presidium of the federal states and the Paul Ehrlich Institute are responsible for this. They only grant licenses if the consistent quality of the stem cells is guaranteed and the production is carried out in such a way that there are no concerns regarding patient safety. Quality control plays an important role here.
Important questions: Does the clinic in its country have a manufacturing permit or a tissue collection permit? Does an international clinic have a permit from the competent national authority? ANOVA IRM in Offenbach has corresponding permits for BMC and secretome since 2018.
Are Allogenic or Autologous Stem Cell Therapies Better?
Compare the products offered. Allogenic products from foreign donors are always associated with more risk than autologous stem cells (donor and recipient are the same person). These greater risks include transmission of pathogens (viruses, bacteria) and allergic reactions. Allogeneic products should only be used if they are immunologically safe or the matching of donor and recipient has been verified.
Important questions: Is the product autologous? If not, how is matching tested? How are the donors tested? Testing in accordance with current regulatory standards includes hepatitis B and C, HIV 1 and 2, and Treponema pallidum (syphillis). Other diseases are usually not tested. Therefore, autologous stem cells are safer in that they cannot transmit infectious diseases from the donor.
How Much Time Does Stem Cell Therapy Take? What is the Duration?
Many patients want to be treated quickly. However, care should be taken as products are only safe if patients are also tested beforehand. For this purpose, blood tests are used, which usually take 1 day. If you are not tested beforehand, you cannot be sure if previous patients have contaminated the premises with bacteria or viruses.
Question for the clinic: what donor selection tests do they do in advance?
Beware of Animal Stem Cells, Fresh Cells and Unclear Products!
Animal stem cell products can be very dangerous and should not be used. Their potential of contamination, infection and allergic reactions is generally high. Always check with the appropriate legal authority in advance. All products that are not clearly and transparently explained should be considered questionable. For all allogenic therapies, ask explicitly about the donors and donor testing, i.e. what the donors were tested for.
We do not Recommend SVF - Stromal Vascular Fraction!
The stromal vascular fraction (SVF) is a product we do not offer. It is obtained from adipose tissue in an isolation procedure lasting approximately 2 hours and contains only a few stem cells. Due to the manual, open isolation process, contamination may occur. Since SVF is applied immediately after isolation, sufficient quality control cannot be performed to detect the potential contamination.
BMC, on the other hand, is isolated in a closed system which is muss less prone to contamination and is much faster. MSC or MSC secretome generated from SVF cells are cultured, which gives the time to perform in-process quality control. The secretome is frozen after production which allows for the highest safety quality control as the end product is analyzed.
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
How do Stem Cells Work? Effect Hypotheses for Stem Cell Treatments
Stem cells are said to have two basic modes of action:
- Immunomodulation (influencing the immune system, mostly inhibiting excessive immune reactions)
- The promotion of tissue regeneration
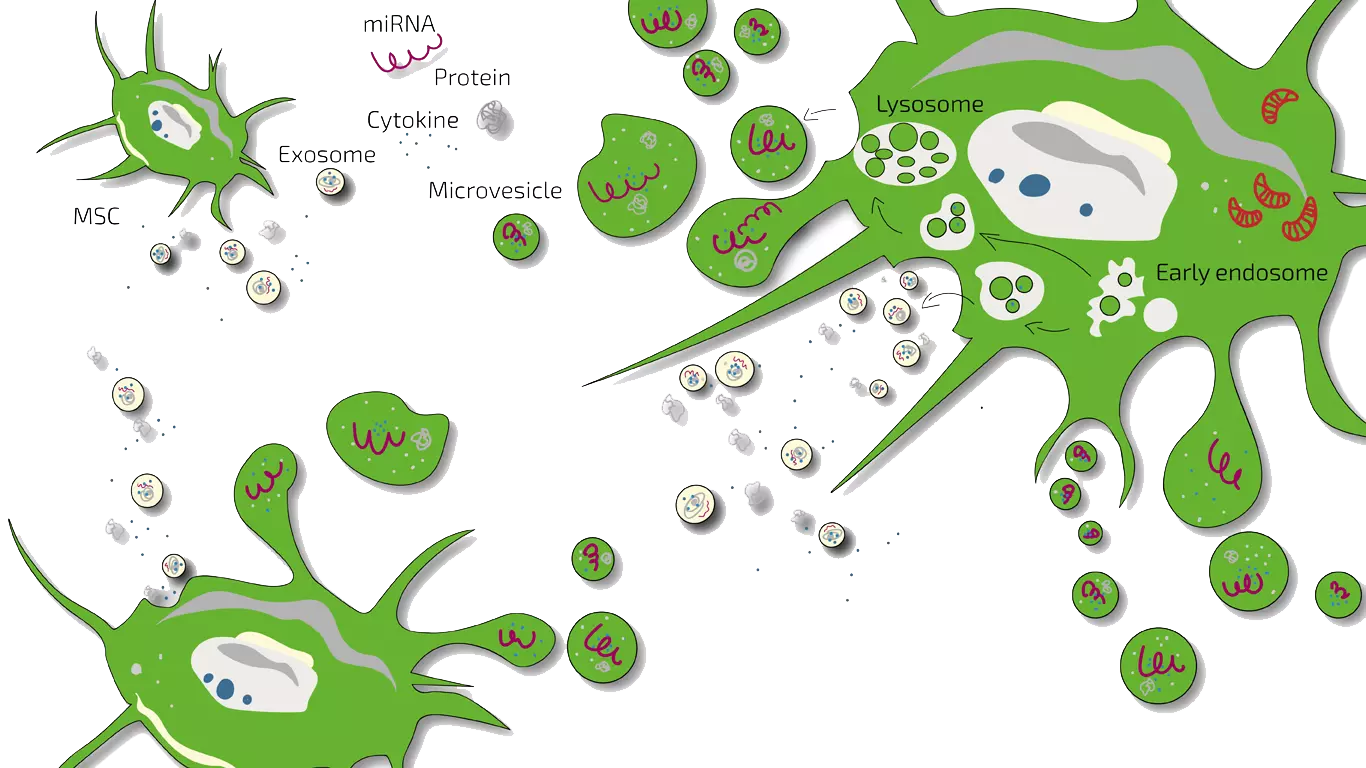
In the early years of stem cell research, it was assumed that stem cells would replace damaged tissue by "integrating" themselves into the tissue. Today, we know that this is not the case. Rather, stem cells act as moderators and motidulators. This means that when stem cells are injected into an inflamed area, they moderate the immune system there, i.e. they inhibit strong inflammation by "communicatively convincing" the triggering immune cells to let the inflammation subside. The communication takes place via messenger substances that are released by the stem cell and either bind to the outside of the immune cell and trigger signals or are taken up by the immune cell and then trigger a signal inside. The "communicators" in their totality are referred to as the secretome, which contains exosomes, microvesicles, cytokines, miRNA (Mirk RNA), and proteins. This moderation is called immunomodulation.
The second effect stem cells can have is to promote regeneration. Stem cells "motivate" the cells in the damaged tissue to divide, regenerate injury or damage, and thus initiate healing. Often, regeneration in damaged tissue is blocked because inflammation is present. Here, stem cells can then exert both effects in succession. The exact way in which stem cells achieve this is not clear, which is why these messengers are not yet available as drugs, but stem cells and their messengers (secretome, exsosomes) are used to treat diseases.
What are Stem Cells?
A stem cell is a very special type of cell that can replicate and develop into many different cell types in the body during early life and growth.
Because of this, they become a repair mechanism of the body. By dividing without limits, they replenish all the other cells. And with each new division, it has the potential to either remain a stem cell or another type of cell with a special function.
Essentially, they can become a muscle cell, a red blood cell, or even a brain cell.
What are Embryonic Stem Cells?
This is a special type of stem cell that is pluripotent. This means that they can change into any type of cell. They are very important because if you’re sick and you need healthy cells for a certain part of your body, then they can replace the unhealthy ones.
However, there have been ethical issues about the method of getting embryonic stem cells. Here at ANOVA, we assure our clients that we only use stem cells which are sourced in a safe and ethical way (not from embryonic sources), obtained from their own tissue.
What are Adult Stem Cells?
Despite their name, they can be found in the bodies of both children and adults. They have the potential to renew the entire tissue from which they come from, with just a few cells.
Why do Adults Have Stem Cells?
Their purpose is to maintain and repair the tissue where they are located. It is part of our body’s self-healing system.
Where are Adult Stem Cells Found?
They are located in many organs and tissues, including the brain, bone marrow, blood, muscle, skin, and many other areas. This is why using adult stem cells is the more popular and more ethical choice for stem cell therapy.
Here at ANOVA, all our available stem cell treatments use mesenchymal stem cells (MSCs), which are easily obtained from fat tissue or from bone marrow. Harvesting stem cells from the patients’ own bodies ensure little to no adverse effects on the health of the patients.
What is the Difference Between Embryonic and Adult Stem Cells?
Embryonic and adult stem cells vary in their abilities. They also differ in number and type of cells they can become. To be more specific, adult stem cells can only transform into several types of cells but embryonic stem cells can become all cell types of the body.
Embryonic stem cells can also be grown in a laboratory, while adult stem cells come from the patient, so they may be harder to grow. However, this makes them less likely to be rejected by the patient’s immune system after the procedure. This is one of the reasons why we have chosen to use adult stem cells at ANOVA.
What are Pluripotent Stem Cells?
Pluripotent is a characteristic of a cell and not a separate cell type. This trait in cells simply means that they can self-replicate and have the ability to develop into other types of cells or tissues.
Also called the “master cells”, they're able to make cells from the different body layers so they can potentially produce cells or tissues the body needs to repair itself.
What are Hematopoietic Stem Cells?
Pluripotent is a characteristic of a cell and not a separate cell type. This trait in cells simply means that they can self-replicate and have the ability to develop into other types of cells or tissues.
It is a subtype of an adult stem cell that originates in the bone marrow. Also called the “blood stem cell”, it can differentiate into all types of blood cells, including white blood cells, red blood cells, and platelets. They are usually found in the bone marrow and in the peripheral blood.
What are Multi-Potent Stem Cells?
They have the power to renew or refresh themselves. After they divide themselves, they can develop into different types of specialized cells. As such, most adult stem cells are multi-potent stem cells.
What are Mesenchymal Stem Cells?
Mesenchymal stem cells, or MSCs, are a type of multipotent stem cell, which can differentiate into a variety of cell types, including fat cells, bone cells, muscle cells, cartilage cells, and more.
As previously mentioned, this is the type of stem cell that we use at ANOVA.
Do Plants Have Stem Cells?
Plants don’t have stem cells similar to humans. However, their cells have a kind of vitality that allows them to provide a steady supply of cells that can form differentiated tissues and organs.
The two characteristics mentioned -- the ability to create mature cell types and the ability to self-renew -- are what make them special. Because of these two qualities, they protect plants and help them withstand the harsh environment and external factors.
Plants are immobile and they can get damaged easily, so their stem cells are their way of protecting themselves.
Why are Stem Cells Important?
They have several uses, both in research and in healing:
- They have the potential to regenerate and repair damaged tissues. Because of this quality, they are used in therapies for people with:
- Spinal cord injuries
- Parkinson's disease
- ALS or amyotrophic lateral sclerosis
- Alzheimer's disease
- Heart disease
- Type 1 diabetes
- Stroke
- Burns
- Osteoarthritis
While there are recorded cases of improvements in patients after therapy, further clinical trials are needed to fully evaluate its effectiveness. - Researchers are also studying them to understand how diseases occur and how to best fight them. By studying how they develop into different kinds of cells, doctors can better understand how diseases start and develop.
- They can also be used to test new medicine and its safety and quality. Because they can replicate other types of cells, they can take on the properties of the types of cells a specific drug aims to target. For example, if a new medicine for heart disease is being tested, then it can be used on stem cells that mature into heart cells. Researchers will find out whether the cells were harmed during the clinical trial of the drug and whether it is safe to administer to patients.
Where do Stem Cells Come From?
Researchers have discovered several sources:
- Embryonic - They come from embryos that are three to five days old.
- Adult - They can be found in tissues such as bone marrow or fat.
- Perinatal - They are located in the amniotic fluid and blood from the umbilical cord. They also have the ability to change into specialized cells.
Who Discovered Stem Cells?
There is an ongoing debate about who discovered them. Their discovery is usually attributed to Canadian scientists Drs. James Till and Ernest McCulloch. However, they were mentioned in scientific journals before Till and McCulloch’s pioneering studies in hematopoietic stem cell research.
When Where They Discovered?
There were many studies that used the term “stem cells” before Till and McCulloch’s 1963 research. The earliest ones date back to the ‘30s and ‘40s.
For example, in a 1932 research paper on the effects of radiation on cells, American scientist Dr. Florence Sabin mentioned that one of the types of cells that can be damaged by radiation are hematopoietic stem cells.
In 1936, the same Dr. Sabin published an article about the relationship of stem cells and white blood cells.
What made Till and McCulloch’s paper different from previous works is that their research focused on hematopoietic stem cells, while others were on stem cells from hematopoietic tumors.
When did Stem Cell Research Take off?
Scientists usually work with two types of stem cells: embryonic and non-embryonic or "adult" stem cells. This started in 1981 when scientists extracted stem cells from mouse embryos. In 1988, this study helped researchers harvest them from human embryos, which are later grown in laboratories.
Roughly two decades later, scientists have reprogrammed specialized adult stem cells genetically to function like a stem cell. These are called induced pluripotent stem cells and this breakthrough paved the way to new research and treatments
How are Embryonic Stem Cells Harvested?
“Harvesting” or the act of getting them can happen in many ways. The way they are harvested affects how they are used in the research or treatment of diseases.
Before, embryonic stem cells were harvested by destroying a human embryo and this became quite controversial. In newer, more advanced techniques, this process was eliminated because it was found that they could be extracted from blastomeres, a cell formed on the outer layer of a fertilized ovum. This avoids the destruction of the embryo.
On the other hand, adult stem cells need to be harvested directly from the source where they would also be used. For example, for the treatment of blood diseases, you would need a blood stem cell. And you can extract them from the bone marrow, where blood is produced.
At ANOVA, we make sure that we harvest stem cells in an ethical and safe way. For all our treatments, we use the patient’s own fat or bone marrow to extract mesenchymal stem cells for our treatments. ANOVA does not apply or employ embryonic stem cells.
How are Mesenchymal Stem Cells Harvested?
Mesenchymal stem cells can be extracted from different sources.
Stem cells from fat tissue are extracted using nutational liposuction, which allows the removal of fat without destroying your cells. At the same time, this minimally invasive procedure only requires local anesthesia and light sedation. Afterwards the fat is transferred into a sterile container for further processing in the laboratory to extract the cell fraction.
Stem cells can also be extracted from bone marrow. This can also be performed using local anesthesia. Red more about Bone Marrow Concentrate here.
What are Stem Cells Used for?
Stem cells are used for both research and therapy.
Of course, one of the most important applications is their use for stem cell-based therapies. Stem cell therapy can be applied for a number of conditions. Read more about it here.
Nowadays, donated organs are used to replace ones that were malfunctioning. However, this process can take longer because of the lack of suitable donors. Stem cells can be a very elegant and efficient alternative, because they can differentiate into specific cell types, which can replace the damaged cells and tissues. The good thing about them is that they are a renewable source and you don’t have to wait for a donor.
As mentioned previously, stem cells can also be used for research purposes. Research in the field of embryonic stem cells, for example, can help us further understand what happens during human development. With the help of research, scientists can identify how stem cells become differentiated cells, which eventually form into the tissues and organs.
Research in this field highly contributes to the ongoing studies on serious medical conditions, such as cancer and birth abnormalities. This is because these diseases are caused by atypical cell division and differentiation. By understanding these conditions better, we are a step closer to finding cures and strategies for therapy.
Stem cells can also be used to test new drugs. For example, new medicines are tested on differentiated cells generated from human pluripotent stem cells.
What do Stem Cells Look Like?
Each cell type has its own size and structure appropriate for its job.
For example, brain cells communicate commands to the body so they’re shaped like webs because they need to reach out to the different types of cells surrounding them.
Even if these cells have different functions, they all consist of the same main components:
- Nucleus - Contains DNA which contains information about the body.
- Cytoplasm - Liquid surrounding the nucleus which contains the materials the cell needs to do its job.
- Cell Membrane - Surface of the cell which sends and receives signals from other cells so they can perform their tasks.
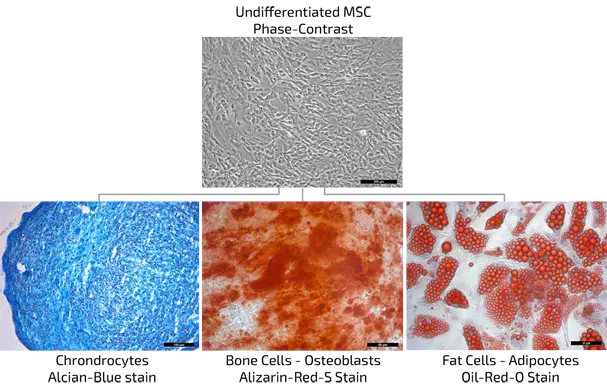
MSC proof of nature by differentiation
How Does Stem Cell Therapy Work?
The way the therapy works depends on the approach. There are two fundamentally different approaches to stem cell therapy.
In the first approach, stem cells are extracted from the source and re-applied to the patient where it is needed. There, stem cells can initiate regeneration and tissue repair.
The source can be fat tissue, bone marrow, or other tissues from the patient’s own body. The location and type of re-application depends on the condition that is being treated.
At ANOVA, we extract bone marrow and re-apply Bone Marrow Concentrate (BMC) containing MSCs to the patient. Read more about it here.
In the second approach, stem cells are also extracted from the same sources, but they are used to produce the stem cell secretome, or stem cell exosomes. In this approach, it is not the cells that are being applied, but the cell-free stem cell secretome.
The secretome is defined as the sum of all soluble molecules (e.g. growth factors and cytokines) that are being released from the cell. These molecules are mainly utilized by the cell to communicate with other cells. They can initiate different mechanism in the surrounding cells.
Why is the stem cell secretome effective? Recent research has found evidence that the main regenerative effects of stem cells descend from these molecules which the stem cells release. Read more about stem cell secretome and its effects here.
How Much Does Stem Cell Therapy Cost?
There are many clinics in North America that advertise stem cell therapy. Some of their treatments can set you back USD 4,000 to USD 7,000.
A report by American media agency PBS also states that in some clinics, treatments for the whole body can cost USD 20,000 to USD 30,000.
In Germany, stem cell treatments can start around 5.000 Euro for single treatements up to some ten-thousand for treatment programs with repeated infusion. Examples of the diseases or conditions requiring this type of treatment include:
- Crohn’s disease
- Multiple sclerosis
- Neurodegenerative diseases, like Alzheimer’s disease and dementia
- Psoriatic or rheumatoid arthritis
Despite these costs, many people in the US or Canada still try them out because it’s comparably cheaper to treatments done in Europe. However, these clinics have been called out due to compliance and ethical issues.
Therefore, before you start any treatment, it’s worth researching the clinic. Take the advice provided by the FDA on stem cell clinics in North America to heart and make sure the clinic you choose is compliant to government regulations. Flying out to Europe may cost more, but in the long run, their high standards and strict compliance increases the chances of effectiveness.
Make sure they are using not just the most advanced but the safest procedures available, to make sure you’re getting your money’s worth.
How much do stem cell injections cost?
Prices vary depending on type of stem cell, type of tissue source, type of treatment, and country. There are clinics in the US and Canada which are reportedly pricing stem cells injections at USD 5,000 to 50.000. In Germany, stem cell treatments start at around 5.000 up to some ten-thousand Euro.
At ANOVA, we consider these injections only a part of the whole therapy program. We strive for long-lasting results and this can only be done if we look at the treatment in a safe, holistic way. Get in touch with us so we can give you an estimate on how much a therapy at ANOVA costs.
What Diseases can Stem Cells Help to Treat?
Stem cell treatment is not a cure-all, but there are many studies and trials which show its effectiveness in certain diseases.
Stroke and Heart Disease
For many survivors of stroke, thrombolysis or the dissolution of the blood clot is the only option available to manage their condition. Thanks to advancements in stem cell research, stroke patients can now use their regenerative and healing power without adverse side effects.
Parkinson’s Disease
A disease affecting millions of people around the globe, it’s unfortunate that Parkinson’s disease currently has no known cure. What many patients do is simply suppress the symptoms by undergoing hormone replacement-based therapies. Unfortunately, this does not stop neural degeneration or replace dead brain cells.
Thankfully, recent clinical trials have shown encouraging results for patients treated with MSCs. Secretome Therapy combined with a personalized treatment program offers a promising alternative to known medications.
Erectile Dysfunction and Impotence
Men of all ages can be affected with erectile dysfunction and reduced potency which are often linked to diabetes and high blood pressure. While there are many pills or alternative treatments advertised to “cure” these conditions, they can only work temporarily and do not address the root cause of the problem.
Stem cell-based therapies have become the alternative, but a medical assessment is essential to start a private and personalized treatment plan, which should include nutrition and lifestyle recommendations.
Osteoarthritis
As the leading cause of pain and disability in the world, osteoarthritis has stopped many people across the world from living a mobile and dynamic life.
Recent evidence-based research has shown that stem cell-based therapies offer longer lasting effects compared to standard medications which only mask the symptoms.
It’s a good thing that the next generation of stem cell-based therapies, including the Secretome Therapy, can possibly replenish lost nerve supply, modulate immune responses of the conditions, and more.
Knee Injuries
Those with knee injuries or knee degeneration, may feel like they have very limited therapy options. However, there is a way for them to get back to their active lifestyle faster, without the hassle of surgery.
There is clinical evidence that shows how cell-based therapies can improve the healing process in knee injuries. Surgery may still be needed in some cases, but combining it with the therapies are considered safe.
Back Pain and Spine Conditions
More often than not, back pain and spine problems are treated using invasive and risky methods like surgery. Others simply rely on pain medications. It may seem like a simple problem, but it affects the patients in all aspects of life. This is why a long-term solution is preferred that addresses the cause of these conditions on a cellular level.
This is where stem cell therapy comes in, which have been proven to be efficient in tackling pain and spinal degenerations. Among the different types of treatments, those that have shown regenerative effects include Bone Marrow Concentrate (BMC), Mesenchymal Stem Cells (MSC) and Platelet Rich Plasma (PRP).
ALS - Amyotrophic Lateral Sclerosis
Also known as motor neuron disease or Lou Gehrig’s disease after a popular baseball player with the same condition, ALS is characterized by severe motor dysfunction because of the loss of nerve stimulation.
There are studies which suggest that stem cell therapy can be a promising new approach to protect the motor neurons of ALS patients.
RA - Rheumatoid Arthritis
Rheumatoid Arthritis (RA) is an autoimmune disease without any cure available. However, the latest stem cell-based therapies can address the problems in three ways -- it can stop inflammations, module the immune responses and regenerate tissues.
Forms of arthritis that can benefit from this include psoriatic arthritis, juvenile idiopathic arthritis, vacuities, and gout.
MS - Multiple Sclerosis
Medications available for multiple sclerosis attempt to slow down its progression but they don't actually reverse them. Because of this, patients with MS have the possibility to turn to stem cell-based therapies, which can stimulate regeneration of damaged brain cells or at least slow down the progress of the disease.
Anti-Aging
With so many anti-aging products on the market, why is stem cell therapy the superior choice? Simply because it’s capable of tissue regeneration on a cellular level. To be more specific, stem cells can revitalize the functions of the body, not just on the surface, but from within!
What are the Side Effects of Stem Cell Therapy?
Although there are reported, such as nausea or vomiting after the treatment, this is dependent on the condition of the patient, the specific type of treatment, and the disease that is being treated.
This is why at ANOVA, we make sure that we do a thorough examination of every patient and tailor fit our therapy to their special needs.
Is it Possible to Increase Stem Cells Naturally?
Like other types of cells, you can have healthy stem cells that regenerate quickly if you have proper nutrition and a healthy lifestyle.
There are also studies which hint at how their production can be boosted like this 2018 Massachusetts of Institute of Technology study which suggests faster regeneration after a 24-hour fast.
There are certainly many new discoveries in the field and here at ANOVA, we are excited about the many possibilities that this can contribute to the constant improvement of our treatments.
If you want to explore your stem cell therapy options with us, we are more than happy to answer your inquiries.
Does Medical Insurance Typically Cover the Therapy?
Treatment programs at the ANOVA Institute for Regenerative Medicine are not covered by health insurance at the moment. They are available only on a self-pay basis.
We are happy to talk to you about your payment options. Feel free to reach out to us by sending an email or calling us.
How can I Donate Stem Cells?
While there are other clinics that receive donations, here at ANOVA, we do not use them on our patients.
For our procedures, we extract the adult stem cells from our own patients’ fat tissue or bone marrow, which guarantees their compatibility with our treatments.
Stem Cell Therapies sorted by stem cell type (source tissue) and product
Further References for MSC, BMC, Stemcell Secretome and EVs
- Georg Hansmann, Philippe Chouvarine, Franziska Diekmann, Martin Giera, Markus Ralser, Michael Mülleder, Constantin von Kaisenberg, Harald Bertram, Ekaterina Legchenko & Ralf Hass "Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension". Nature Cardiovascular Research volume 1, pages568–576 (2022).
- Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis . Arthritis Rheum. 2003;48:3464–74.
- Lee KB, Hui JH, Song IC, Ardany L, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cell. 2007;25:2964–71.
- Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy . 2009;25(12):1391–400.
- Black L, Gaynor J, Adams C, et al. Effect of intra-articular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200.
- Centeno C, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–53.
- Centeno C, Kisiday J, Freeman M, et al. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: a case study. Pain Physician. 2006;9:253–6.
- Centeno C, Schultz J, Cheever M. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell. 2011;5(1):81–93.
- Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose derived stem cells: a case series. J Med Case Rep. 2001;5:296.
- Kuroda R, Ishida K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31.
- Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422–8.
- Saw KY et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94.
- Vangsness CT, Farr J, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg. 2014;96(2):90–8.
- Freitag, Julien, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy–a review. BMC musculoskeletal disorders 17.1 (2016): 230.
- Maumus, Marie, Christian Jorgensen, and Danièle Noël. " Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. " Biochimie 95.12 (2013): 2229-2234.
- Dostert, Gabriel, et al. " How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication?. " Frontiers in Cell and Developmental Biology 5 (2017).
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- Toh, Wei Seong, et al. " MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. " Seminars in Cell & Developmental Biology. Academic Press, 2016.
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- S. Koelling, J. Kruegel, M. Irmer, J.R. Path, B. Sadowski, X. Miro, et al., Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis , Cell Stem Cell 4 (2009) 324–335.
- B.A. Jones, M. Pei, Synovium-Derived stem cells: a tissue-Specific stem cell for cartilage engineering and regeneration , Tissue Eng. B: Rev. 18 (2012) 301–311.
- W. Ando, J.J. Kutcher, R. Krawetz, A. Sen, N. Nakamura, C.B. Frank, et al., Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage, Cytotherapy 16 (2014) 776–788.
- K.B.L. Lee, J.H.P. Hui, I.C. Song, L. Ardany, E.H. Lee, Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model, Stem Cells 25 (2007) 2964–2971.
- W.-L. Fu, C.-Y. Zhou, J.-K. Yu, A new source of mesenchymal stem cells for articular cartilage repair: mSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model , Am. J. Sports Med. 42 (2014) 592–601.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, et al., Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration , Biomaterials 33 (2012) 7008–7018.
- E.-R. Chiang, H.-L. Ma, J.-P. Wang, C.-L. Liu, T.-H. Chen, S.-C. Hung, Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits , PLoS One 11 (2016) e0149835.
- H. Nejadnik, J.H. Hui, E.P. Feng Choong, B.-C. Tai, E.H. Lee, Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study , Am. J. Sports Med. 38 (2010) 1110–1116.
- I. Sekiya, T. Muneta, M. Horie, H. Koga, Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects , Clin. Orthop. Rel. Res. 473 (2015) 2316–2326.
- Y.S. Kim, Y.J. Choi, Y.G. Koh, Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes , Am. J. Sports Med. 43 (2015) 2293–2301.
- W.-L. Fu, Y.-F. Ao, X.-Y. Ke, Z.-Z. Zheng, X. Gong, D. Jiang, et al., Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment , Knee 21 (2014) 609–612.
- Y.-G. Koh, O.-R. Kwon, Y.-S. Kim, Y.-J. Choi, D.-H. Tak, Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial , Arthrosc. J. Arthrosc. Relat. Surg. 32 (2016) 97–109.
- T.S. de Windt, L.A. Vonk, I.C.M. Slaper-Cortenbach, M.P.H. van den Broek, R. Nizak, M.H.P. van Rijen, et al., Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-Stage cartilage repair in humans upon mixture with recycled autologous chondrons , Stem Cells (2016) (n/a-n/a).
- L. da Silva Meirelles, A.M. Fontes, D.T. Covas, A.I. Caplan, Mechanisms involved in the therapeutic properties of mesenchymal stem cells , Cytokine Growth Factor Rev. 20 (2009) 419–427.
- W.S. Toh, C.B. Foldager, M. Pei, J.H.P. Hui, Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration , Stem Cell Rev. Rep. 10 (2014) 686–696.
- R.C. Lai, F. Arslan, M.M. Lee, N.S.K. Sze, A. Choo, T.S. Chen, et al., Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury , Stem Cell Res. 4 (2010) 214–222.
- S. Zhang, W.C. Chu, R.C. Lai, S.K. Lim, J.H.P. Hui, W.S. Toh, Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration, Osteoarthr . Cartil. 24 (2016) 2135–2140.
- S. Zhang, W. Chu, R. Lai, J. Hui, E. Lee, S. Lim, et al., 21 – human mesenchymal stem cell-derived exosomes promote orderly cartilage regeneration in an immunocompetent rat osteochondral defect model , Cytotherapy 18 (2016) S13.
- C.T. Lim, X. Ren, M.H. Afizah, S. Tarigan-Panjaitan, Z. Yang, Y. Wu, et al., Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model
- A. Gobbi, G. Karnatzikos, S.R. Sankineani, One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee , Am. J. Sports Med. 42 (2014) 648–657.
- A. Gobbi, C. Scotti, G. Karnatzikos, A. Mudhigere, M. Castro, G.M. Peretti, One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years , Knee Surg. Sports Traumatol. Arthrosc. (2016) 1–8.
- A. Gobbi, G. Karnatzikos, C. Scotti, V. Mahajan, L. Mazzucco, B. Grigolo, One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-Year follow-up , Cartilage 2 (2011) 286–299.
- K.L. Wong, K.B.L. Lee, B.C. Tai, P. Law, E.H. Lee, J.H.P. Hui, Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up , Arthrosc. J. Arthrosc. Relat. Surg. 29 (2013) 2020–2028.
- J.M. Hare, J.E. Fishman, G. Gerstenblith, et al., Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the poseidon randomized trial, JAMA 308 (2012) 2369–2379.
- L. Wu, J.C.H. Leijten, N. Georgi, J.N. Post, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation , Tissue Eng. A 17 (2011) 1425–1436.
- L. Wu, H.-J. Prins, M.N. Helder, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells in chondrocyte Co-Cultures are independent of culture conditions and cell sources , Tissue Eng. A 18 (2012) 1542–1551.
- S.K. Sze, D.P.V. de Kleijn, R.C. Lai, E. Khia Way Tan, H. Zhao, K.S. Yeo, et al., Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells , Mol. Cell. Proteomics 6 (2007) 1680–1689.
- M.B. Murphy, K. Moncivais, A.I. Caplan, Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine , Exp. Mol. Med. 45 (2013) e54.
- M.J. Lee, J. Kim, M.Y. Kim, Y.-S. Bae, S.H. Ryu, T.G. Lee, et al., Proteomic analysis of tumor necrosis factor--induced secretome of human adipose tissue-derived mesenchymal stem cells , J. Proteome Res. 9 (2010) 1754–1762.
- S. Bruno, C. Grange, M.C. Deregibus, R.A. Calogero, S. Saviozzi, F. Collino, et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury, J. Am. Soc. Nephrol. 20 (2009) 1053–1067.
- M. Yá˜nez-Mó, P.R.-M. Siljander, Z. Andreu, A.B. Zavec, F.E. Borràs, E.I. Buzas, et al. Biological properties of extracellular vesicles and their physiological functions (2015).
- C. Lawson, J.M. Vicencio, D.M. Yellon, S.M. Davidson, Microvesicles and exosomes: new players in metabolic and cardiovascular disease , J. Endocrinol. 228 (2016) R57–R71.
- A.G. Thompson, E. Gray, S.M. Heman-Ackah, I. Mager, K. Talbot, S.E. Andaloussi, et al., Extracellular vesicles in neurodegenerative diseas—pathogenesis to biomarkers, Nat. Rev. Neurol. 12 (2016) 346–357.
- I.E.M. Bank, L. Timmers, C.M. Gijsberts, Y.-N. Zhang, A. Mosterd, J.-W. Wang, et al., The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease , Expert Rev. Mol. Diagn. 15 (2015) 1577–1588.
- T. Kato, S. Miyaki, H. Ishitobi, Y. Nakamura, T. Nakasa, M.K. Lotz, et al., Exosomes from IL-1 stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes , Arthritis. Res. Ther. 16 (2014) 1–11.
- R.W.Y. Yeo, S.K. Lim, Exosomes and their therapeutic applications, in: C. Gunther, A. Hauser, R. Huss (Eds.), Advances in Pharmaceutical Cell TherapyPrinciples of Cell-Based Biopharmaceuticals, World Scientific, Singapore, 2015, pp. 477–491.
- X. Qi, J. Zhang, H. Yuan, Z. Xu, Q. Li, X. Niu, et al., Exosomes secreted by human-Induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats , Int. J. Biol. Sci. 12 (2016) 836–849.
- R.C. Lai, F. Arslan, S.S. Tan, B. Tan, A. Choo, M.M. Lee, et al., Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles , J. Mol. Cell. Cardiol. 48 (2010) 1215–1224.
- Y. Zhou, H. Xu, W. Xu, B. Wang, H. Wu, Y. Tao, et al., Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro , Stem Cell Res. Ther. 4 (2013) 1–13.
- Y. Qin, L. Wang, Z. Gao, G. Chen, C. Zhang, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo , Sci. Rep. 6 (2016) 21961.
- M. Nakano, K. Nagaishi, N. Konari, Y. Saito, T. Chikenji, Y. Mizue, et al., Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes , Sci. Rep. 6 (2016) 24805.
- K. Nagaishi, Y. Mizue, T. Chikenji, M. Otani, M. Nakano, N. Konari, et al., Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes , Sci. Rep. 6 (2016) 34842.
- S.R. Baglio, K. Rooijers, D. Koppers-Lalic, F.J. Verweij, M. Pérez Lanzón, N. Zini, et al., Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species , Stem Cell Res. Ther. 6 (2015) 1–20.
- T. Chen, R. Yeo, F. Arslan, Y. Yin, S. Tan, Efficiency of exosome production correlates inversely with the developmental maturity of MSC donor, J. Stem Cell Res. Ther. 3 (2013) 2.
- R.C. Lai, S.S. Tan, B.J. Teh, S.K. Sze, F. Arslan, D.P. de Kleijn, et al., Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome , Int. J. Proteomics 2012 (2012) 971907.
- T.S. Chen, R.C. Lai, M.M. Lee, A.B.H. Choo, C.N. Lee, S.K. Lim, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs , Nucleic Acids Res. 38 (2010) 215–224.
- R.W. Yeo, R.C. Lai, K.H. Tan, S.K. Lim, Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell, J. Circ. Biomark. 1 (2013) 7.
- R.C. Lai, R.W. Yeo, S.K. Lim, Mesenchymal stem cell exosomes, Semin. Cell Dev. Biol. 40 (2015) 82–88.
- B. Zhang, R.W. Yeo, K.H. Tan, S.K. Lim, Focus on extracellular vesicles: therapeutic potential of stem cell-derived extracellular vesicles , Int. J. Mol. Sci. 17 (2016) 174.
- Hu G-w, Q. Li, X. Niu, B. Hu, J. Liu, Zhou S-m, et al., Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice , Stem Cell Res. Ther. 6 (2015) 1–15.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li, et al., Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis , J. Transl. Med. 13 (2015) 1–14.
- B. Zhang, M. Wang, A. Gong, X. Zhang, X. Wu, Y. Zhu, et al., HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing, Stem Cells 33 (2015) 2158–2168.
- B. Zhang, Y. Yin, R.C. Lai, S.S. Tan, A.B.H. Choo, S.K. Lim, Mesenchymal stem cells secrete immunologically active exosomes , Stem Cells Dev. 23 (2013) 1233–1244.
- C.Y. Tan, R.C. Lai, W. Wong, Y.Y. Dan, S.-K. Lim, H.K. Ho, Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models , Stem Cell Res. Ther. 5 (2014) 1–14.
- C. Lee, S.A. Mitsialis, M. Aslam, S.H. Vitali, E. Vergadi, G. Konstantinou, et al., Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension , Circulation 126 (2012) 2601–2611.
- B. Yu, H. Shao, C. Su, Y. Jiang, X. Chen, L. Bai, et al., Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1 , Sci. Rep. 6 (2016) 34562.
- Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof of concept clinical trial. Stem Cells. 2014;32(5):1254–66.
- Vega, Aurelio, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
- Davatchi F, Sadeghi-Abdollahi B, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–5
- Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case- controlled study. Int Orthop. 2014;38(9):1811–1818
- Galli D, Vitale M, Vaccarezza M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:6.
- Beitzel K, Solovyova O, Cote MP, et al. The future role of mesenchymal Stem cells in The management of shoulder disorders . Arthroscopy. 2013;29(10):1702–1711.
- Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190.
- Malda, Jos, et al. " Extracellular vesicles [mdash] new tool for joint repair and regeneration. " Nature Reviews Rheumatology (2016).
Further References about PRP
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4(1):52–62.
Extras
- Xu, Ming, et al. " Transplanted senescent cells induce an osteoarthritis-like condition in mice. " The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (2016): glw154.
- McCulloch, Kendal, Gary J. Litherland, and Taranjit Singh Rai. " Cellular senescence in osteoarthritis pathology ." Aging Cell (2017).
Patient Services at ANOVA Institute for Regenerative Medicine
- Located in the center of Germany, quick access by car or train from anywhere in Europe
- Simple access worldwide, less than 20 minutes from Frankfurt Airport
- Individualized therapy with state-of-the-art stem cell products
- Individually planned diagnostic work-up which include world-class MRI and CT scans
- German high quality standard on safety and quality assurance
- Personal service with friendly, dedicated Patient Care Managers
- Scientific collaborations with academic institutions to assure you the latest regenerative medical programs