Stem Cell Therapy for Amyotrophic Lateral Sclerosis
at ANOVA IRM in Offenbach, Germany
Amyotrophic Lateral Sclerosis (ALS) is caused by the progressive death of cerebral (upper) and spinal (lower) motor neurons. It is a complex disease which involves the activation of several cellular pathways in both neurons and glial cells. This results in a severe motor dysfunction muscles become atrophic due to the lack of nerve stimulation. Often, the origin of the disease is unknown. ALS is not a typical autoimmune disorder, since autoimmune and inflammatory abnormalities are not the cause of the disease, even though they influence its progression.
Stem Cell-based therapies may be the answer. A study by Mazzini et al. demonstrated that the procedure of ex vivo expansion of autologous Mesenchymal Stem Cells (MSCs) and transplantation into the spinal cord of humans was safe and well tolerated by ALS patients. A more recent study, by the same group, however, confirmed that MSC transplantation into the spinal cord of ALS patients is not only safe, but might also serve as a treatment option for future cell-based clinical trials for the treatment of ALS.
Many current pre-clinical studies suggest that stem cell transplantation has the best effect when aimed towards protecting, rather than replacing or repairing the motor neurons of ALS patients. Our experience shows that Stem Cell Secretome is a promising, novel strategy that appears to be more effective and safer than the cells themselves. Stem Cell Secretome is the essence of stem cells, and can facilitate neuro-protection and recovery of neuro-motorial function. ANOVA offers this experimental and novel stem cell-based therapy patients with ALS. For more information, please feel free to contact us.
Amyotrophic Lateral Sclerosis
Diagnostics - Treatment - Medication - Stem Cell Therapies
On this page we inform you about ALS covering an overview on important aspects of causes, treatment options, precision diagnostics as well as our stem cell-based therapies that we offer in Offenbach (near Frankfurt am Main airport), Germany.
Jump directly to the following topics:
- Conventional therapies
- ANOVA therapies for ALS
- Expectations and limits
- Our treatment options
- Workflow of the treatment process
- Sources and Literature
Conventional ALS Therapies vs. Stem Cell Therapy
The therapeutic strategy used for treating ALS is aimed at protecting neurons from degeneration, and to stimulate cell regeneration. However, currently there is no drug treatment that can restore neural cells.
Conventional Therapies are e.g.:
- drugs to relieve painful muscle cramps
- drugs inhibits excessive salivation production
- heat or whirlpool therapy to reduce muscle cramping
- Physiotherapy to retain as much muscle strength as possible
- Physiotherapy to reduce muscle stiffness and cramps
- Lymph drainage
- Nutritional supplementation and healthy diets
- Speech therapy
Recently, the US FDA approved Rilutek®. It is the first drug that has prolonged the survival of ALS patients. It failed, however, to stimulate regeneration or recovery from established damage.
Effects of ALS in a MRI image
ANOVA IRM Germany
Stem cell research has allowed ANOVA, a German Stem Cell Clinic in the heart of Europe near Frankfurt/Main airport, to offer a novel treatment with a new therapeutical approach: The ANOVA Stem Cell Secretome is a cell free and promising treatment option for ALS.
Call us today, whether you wish to apply for a treatment, or simply receive more information.
Stem Cell Treatments for Early ALS at
ANOVA Institute for Regenerative Medicine - Offenbach, Germany
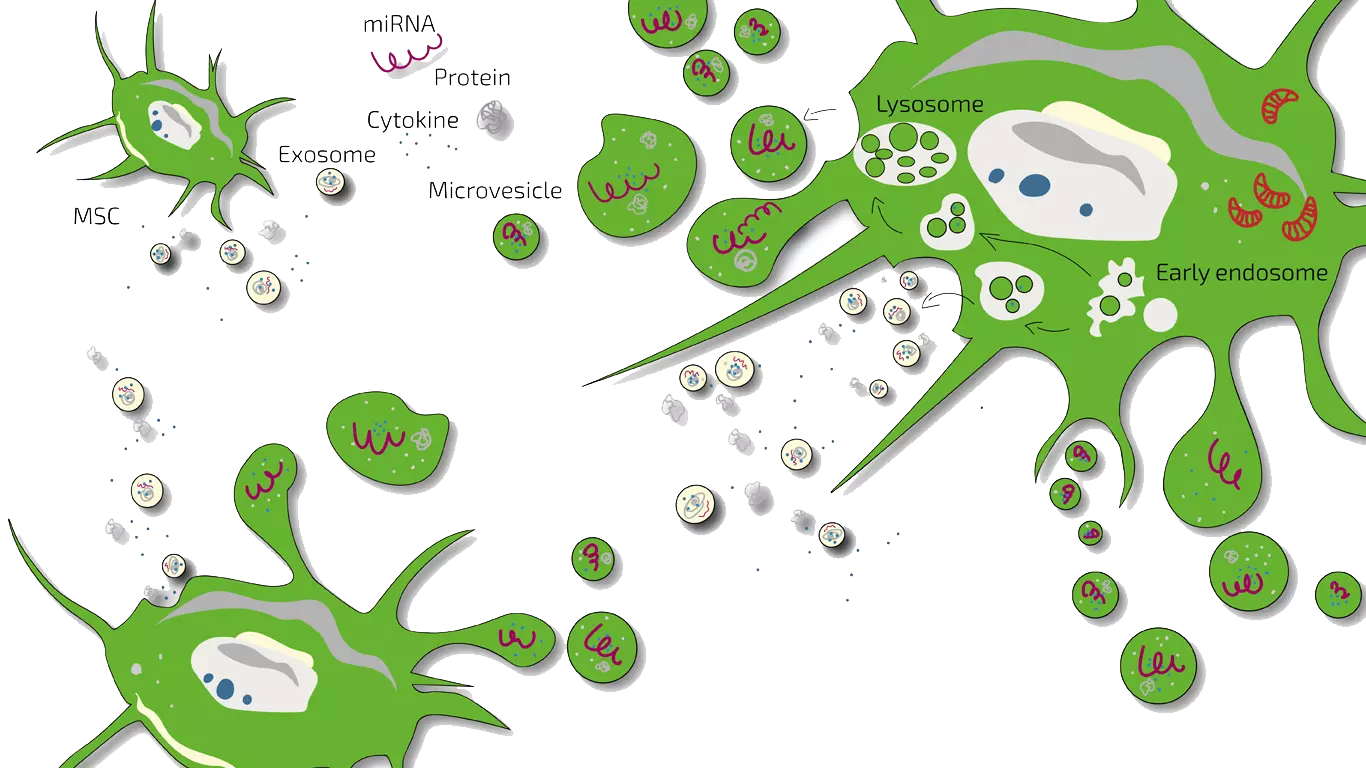
Secretome/Exosomes of MSC
Potency Hypothesis of Stem Cell Therapies
Stem cells possess the potential to communicate with the immune cells that elicit inflammation and by natural, so far not understood mechanisms may inhibit this immune-over-reaction. Furthermore, stem cells have the ability to stimulate regeneration of tissue thereby counteracting the loss of function.
MSEC - Mesenchymal Stem Cell Secretome - Exosomes - Autologous
As ALS is a chronic, so far not curable disease, we on-goingly treat patients with early to mid-stage ALS with MSEC (secretome, exosomes, EVs) of mesenchymal stem cells (MSC, AD-MSC, adipose-derived, fat-derived stem cells) which we harvest from the patients belly in a mini-liposuction (very brief and limited liposuction) under slight sedation. Worldwide, ANOVA is the first stem cell clinic to acquire legal permission form the responsible governmental authorities and therefore, offers high quality, safe and legally-controlled autologous (own) exosome-containing secretome.
The main advantage of MSEC is that in contrast to live stem cells which would loose their therapeutic potency, can be frozen without loss of exosomes. This enables us to produce 10-20 injection doses from one liposuction which can then be administered over a longer treatment period. This is especially advantageous for repeated stimulation of cell survival and regeneration in ALS. What a Secretome/Exosome is and how they compare is explained on our overview page.
Please note that this treatment is not a cure but as any stem cell treatment an experimental, potentially disease-modifying therapy. It requires regular and repeated travelling to Offenbach, Germany.
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
Therapy Workflow for Early to mid-Stage ALS
The precise workflow is described in detail on the stem cell- specific pages of BMC, Secretome/Exosomes and PRP (as combination therapy).
All therapies are divided into phases such as evaluation of the medical history (we analyze your current therapies and medical records), initial counseling and evaluation of potential, patient-individual benefit of a stem cell therapy (indication statement), preliminary examinations, diagnostics, consultation on all therapy options, preparation of an individual treatment plan including cost estimate, harvesting of tissue, production of the stem cell product, quality control of the product and application.
Unfortunately, we only treat patients in an early to mid-stage of ALS. Patients have to breathe unassitedly and have to be fit for the sedation and brief intervention necessary to harvest fat tissue. And according to the risk-benefit ratio, we cannot treat children or pregnant women. In addition, other factors can also be exclusion criteria.
How Long Does a Stem Cell Therapy Take?
The initial analyses and counseling can be done without you having to travel to Offenbach (near Frankfurt/Main, Germany). This period can be 2 weeks up to months depending on the availability of patients slots. If you live further away, we will conduct the initial discussions by telephone or video conference. For the actual treatment, you will travel to Offenbach.
Secretome/Exosome-therapy:
Preparation and harvest of the fat (mini-liposuction) need once 2 days (consecutive days) in Offenbach, followed by enrichment of the mesenchymal stem cells (Secretome/Exosome) and quality control. Approximately 4 weeks after the isolation, the therapy begins according to the therapy plan determined with you. You will then come to Offenbach am Main (Germany) in regular intervals for the application. Depending on where you live and your traveling capacity and restrictions the treatment pattern is adjusted to your needs and abilities. The shelf life of the secretome (exosomes) is 2 years. As ALS is not curable with any treatment, we recommend a double-lipo which produces 20 doses for a continuous treatment over 2 years. Thereafter, a new liposuction has to be performed.
How much Does Stem Cell Treatment Cost?
Our treatments are always tailored to your specific situation, disease, stage and other factors. The therapies differ in the product used (BMC, secretome, PRP or hyaluronic acid), the frequency of treatment as well as the further examinations and your sedation and anesthesia wishes. A treatment for ALS will cost well above ten thousand euros. You will receive a cost estimate for all treatments in advance so that you can accurately estimate what a treatment would cost in your individual case.
Does my Health Insurance Cover the Therapy Costs?
Unfortunately, at the moment it is assumed that health insurance companies do not cover the costs of experimental therapies (BMC, secretome, PRP, micro-fracture technique), i.e. you will have to bear the costs entirely yourself.
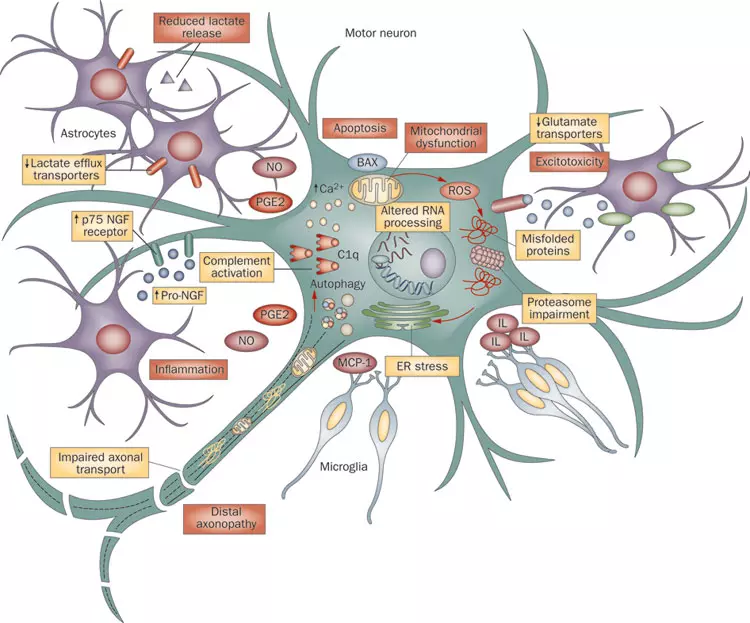
Understanding ALS on a Cellular Level
This figure shows the complexity of ALS, which involves many different pathways in motor neurons and neighboring glia. Microglia (bottom part) activate an inflammatory cascade via MCP-1 secretion. Astrocytes (purple cells) contribute to the injury of motor neurons through various mechanisms, including release of inflammatory mediators such as NO and PGE2 (left), reduced expression and activity of the glutamate transporter (right), reduced lactate release (top left) and activation of pro-NGF–p75 receptor signaling (left). Motor neurons also undergo abnormal RNA processing which, together with overproduction of reactive oxygen species (ROS), contribute to protein mis-folding (center). Mis-folded proteins can form aggregates, leading to cellular stress and ultimately activate autophagy and apoptotic pathways. Two major components of motor neuron injury are mitochondrial impairment and dysregulation of calcium handling (top middle) which also stimulate the apoptotic cascade. Impaired axonal transport (left bottom) may contribute to an energy deficit, disturbing normal functionality (distal axonopathy). Abbreviations: EAAT2, excitatory amino acid transporter 2; ER, endoplasmic reticulum; IL, interleukin; MCP-1, monocyte chemo-attractant protein 1; NGF, nerve growth factor; NO, nitric oxide; PGE2, prostaglandin E2.
Quelle: By Jmarchn-Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=95036158
Stem Cell Secretome Therapy for ALS
Several clinical trials with stem cells for ALS are ongoing. As usual during studies, new insights emerge. The ANOVA Stem Cell Secretome is the product of the latest insights to what actually causes the stem cell therapies to be effective. Stem Cell Secretome for ALS employs mesenchymal stem cells (MSC) secretome for a variety of reasons.
As explained in detail in our Information for Professionals section, MSCs can secrete many trophic and neuro-protective factors. Additionally, among the micro vesicles secreted from the MSC, are the very important exosomes transporting microRNAs (miR-29a, miR-9, miR-124, miR-145). It is known that the exposure of neurons and astrocytes with MSC secreted exosomes leads to an increase of miR-133b which was shown to promote functional neurological recovery.
The ANOVA Stem Cell Secretome harnesses these and many other factors in a high concentration.
It is important to note that novel therapies such as stem cell-based therapies have not undergone the full clinical evaluation yet. Therefore, the attending physician must assess the risks and benefits associated with stem cell therapy for each patient individually. If the benefits outweigh the potential risks, the doctor may suggest experimental therapies to the patient.
References and Literature - Stem Cell-based Therapies for
Amythrophic Aateral Sclerosis-ALS
- Wijesekera, Lokesh C., and P. Nigel Leigh. "Amyotrophic lateral sclerosis." Orphanet journal of rare diseases 4.1 (2009): 3.
- Ferraiuolo, Laura, et al. "Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis." Nature Reviews Neurology 7.11 (2011): 616-630.
- Mazzini, Letizia, et al. "Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans." Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 4.3 (2003): 158-161.
- Mazzini, L., et al. "Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial." Experimental neurology 223.1 (2010): 229-237.
- Papadeas, Sophia T., and Nicholas J. Maragakis. "Advances in stem cell research for Amyotrophic Lateral Sclerosis." Current opinion in Biotechnology 20.5 (2009): 545-551.
- Janson, C. G., et al. "Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis." Journal of hematotherapy & stem cell research 10.6 (2001): 913-915.
- Thomsen, Gretchen M., et al. "The past, present and future of stem cell clinical trials for ALS." Experimental neurology 262 (2014): 127-137
- Staff, Nathan P., et al. "Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS." Neurology 87.21 (2016): 2230-2234.
- Oh, Ki-Wook, et al. "Phase I Trial of Repeated Intrathecal Autologous Bone Marrow Derived Mesenchymal Stromal Cells in Amyotrophic Lateral Sclerosis." Stem cells translational medicine 4.6 (2015): 590-597.
- Petrou, Panayiota, et al. "Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials." JAMA neurology 73.3 (2016): 337-344.
- Farinazzo, Alessia, et al. "Murine adipose-derived mesenchymal stromal cell vesicles: in vitro clues for neuroprotective and neuroregenerative approaches." Cytotherapy 17.5 (2015): 571-578.
- Bonafede, Roberta, et al. "Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis." Experimental cell research 340.1 (2016): 150-158.
- Boruczkowski, D., et al. "Mesenchymal Stem Cells As A Therapeutic Option For Patients With ALS." Gen Med (Los Angel) 4.235 (2016): 2.
Literature and References HAL training for ALS treatment
- Nakajima T, Sankai Y, Takata S, Kobayashi Y, Ando Y, Nakagawa M, Saito T, Saito K, Ishida C, Tamaoka A, Saotome T, Ikai T, Endo H, Ishii K, Morita M, Maeno T, Komai K, Ikeda T, Ishikawa Y, Maeshima S, Aoki M, Ito M, Mima T, Miura T, Matsuda J, Kawaguchi Y, Hayashi T, Shingu M, Kawamoto H. Orphanet J Rare Dis. 2021 Jul 7;16(1):304. PMID: 34233722 Free PMC article. Clinical Trial. https://doi/10.1186/s13023-021-01928-9. RESULTS: We conducted an open-label, randomised, controlled crossover trial to test HAL at nine hospitals between March 6, 2013 and August 8, 2014. ...Cybernic treatment with HAL resulted in a 10.066% significantly improved distance in 2MWT (95% confidence interval, …
- Morioka H, Hirayama T, Sugisawa T, Murata K, Shibukawa M, Ebina J, Sawada M, Hanashiro S, Nagasawa J, Yanagihashi M, Uchi M, Kawabe K, Washizawa N, Ebihara S, Nakajima T, Kano O. J Clin Neurosci. 2022 Mar 10;99:158-163. Online ahead of print. PMID: 35279589 Free article. https://doi.org/10.1016/j.jocn.2022.02.032. We used HAL for patients with amyotrophic lateral sclerosis (ALS) to determine whether HAL training had an effect on their gait ability. ...The 10-meter walk test showed significantly improved cadence, although gait speed, step length on the 10-m walk, or ADL …
- Sczesny-Kaiser M, Kowalewski R, Schildhauer TA, Aach M, Jansen O, Grasmücke D, Güttsches AK, Vorgerd M, Tegenthoff M. Front Neurosci. 2017 Aug 8;11:449. eCollection 2017. PMID: 28848377 Free PMC article. https://doi.org/10.3389/fnins.2017.00449. Recent studies have shown that the voluntary-driven exoskeleton HAL (hybrid assistive limb) can improve walking functions in spinal cord injury and stroke. The aim of this study was to assess safety and effects on walking function of HAL supported treadmill therapy …
Further References for MSC, BMC, Stemcell Secretome and EVs
- Georg Hansmann, Philippe Chouvarine, Franziska Diekmann, Martin Giera, Markus Ralser, Michael Mülleder, Constantin von Kaisenberg, Harald Bertram, Ekaterina Legchenko & Ralf Hass "Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension". Nature Cardiovascular Research volume 1, pages568–576 (2022).
- Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis . Arthritis Rheum. 2003;48:3464–74.
- Lee KB, Hui JH, Song IC, Ardany L, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cell. 2007;25:2964–71.
- Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy . 2009;25(12):1391–400.
- Black L, Gaynor J, Adams C, et al. Effect of intra-articular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200.
- Centeno C, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–53.
- Centeno C, Kisiday J, Freeman M, et al. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: a case study. Pain Physician. 2006;9:253–6.
- Centeno C, Schultz J, Cheever M. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell. 2011;5(1):81–93.
- Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose derived stem cells: a case series. J Med Case Rep. 2001;5:296.
- Kuroda R, Ishida K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31.
- Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422–8.
- Saw KY et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94.
- Vangsness CT, Farr J, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg. 2014;96(2):90–8.
- Freitag, Julien, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy–a review. BMC musculoskeletal disorders 17.1 (2016): 230.
- Maumus, Marie, Christian Jorgensen, and Danièle Noël. " Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. " Biochimie 95.12 (2013): 2229-2234.
- Dostert, Gabriel, et al. " How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication?. " Frontiers in Cell and Developmental Biology 5 (2017).
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- Toh, Wei Seong, et al. " MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. " Seminars in Cell & Developmental Biology. Academic Press, 2016.
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- S. Koelling, J. Kruegel, M. Irmer, J.R. Path, B. Sadowski, X. Miro, et al., Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis , Cell Stem Cell 4 (2009) 324–335.
- B.A. Jones, M. Pei, Synovium-Derived stem cells: a tissue-Specific stem cell for cartilage engineering and regeneration , Tissue Eng. B: Rev. 18 (2012) 301–311.
- W. Ando, J.J. Kutcher, R. Krawetz, A. Sen, N. Nakamura, C.B. Frank, et al., Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage, Cytotherapy 16 (2014) 776–788.
- K.B.L. Lee, J.H.P. Hui, I.C. Song, L. Ardany, E.H. Lee, Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model, Stem Cells 25 (2007) 2964–2971.
- W.-L. Fu, C.-Y. Zhou, J.-K. Yu, A new source of mesenchymal stem cells for articular cartilage repair: mSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model , Am. J. Sports Med. 42 (2014) 592–601.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, et al., Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration , Biomaterials 33 (2012) 7008–7018.
- E.-R. Chiang, H.-L. Ma, J.-P. Wang, C.-L. Liu, T.-H. Chen, S.-C. Hung, Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits , PLoS One 11 (2016) e0149835.
- H. Nejadnik, J.H. Hui, E.P. Feng Choong, B.-C. Tai, E.H. Lee, Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study , Am. J. Sports Med. 38 (2010) 1110–1116.
- I. Sekiya, T. Muneta, M. Horie, H. Koga, Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects , Clin. Orthop. Rel. Res. 473 (2015) 2316–2326.
- Y.S. Kim, Y.J. Choi, Y.G. Koh, Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes , Am. J. Sports Med. 43 (2015) 2293–2301.
- W.-L. Fu, Y.-F. Ao, X.-Y. Ke, Z.-Z. Zheng, X. Gong, D. Jiang, et al., Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment , Knee 21 (2014) 609–612.
- Y.-G. Koh, O.-R. Kwon, Y.-S. Kim, Y.-J. Choi, D.-H. Tak, Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial , Arthrosc. J. Arthrosc. Relat. Surg. 32 (2016) 97–109.
- T.S. de Windt, L.A. Vonk, I.C.M. Slaper-Cortenbach, M.P.H. van den Broek, R. Nizak, M.H.P. van Rijen, et al., Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-Stage cartilage repair in humans upon mixture with recycled autologous chondrons , Stem Cells (2016) (n/a-n/a).
- L. da Silva Meirelles, A.M. Fontes, D.T. Covas, A.I. Caplan, Mechanisms involved in the therapeutic properties of mesenchymal stem cells , Cytokine Growth Factor Rev. 20 (2009) 419–427.
- W.S. Toh, C.B. Foldager, M. Pei, J.H.P. Hui, Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration , Stem Cell Rev. Rep. 10 (2014) 686–696.
- R.C. Lai, F. Arslan, M.M. Lee, N.S.K. Sze, A. Choo, T.S. Chen, et al., Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury , Stem Cell Res. 4 (2010) 214–222.
- S. Zhang, W.C. Chu, R.C. Lai, S.K. Lim, J.H.P. Hui, W.S. Toh, Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration, Osteoarthr . Cartil. 24 (2016) 2135–2140.
- S. Zhang, W. Chu, R. Lai, J. Hui, E. Lee, S. Lim, et al., 21 – human mesenchymal stem cell-derived exosomes promote orderly cartilage regeneration in an immunocompetent rat osteochondral defect model , Cytotherapy 18 (2016) S13.
- C.T. Lim, X. Ren, M.H. Afizah, S. Tarigan-Panjaitan, Z. Yang, Y. Wu, et al., Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model
- A. Gobbi, G. Karnatzikos, S.R. Sankineani, One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee , Am. J. Sports Med. 42 (2014) 648–657.
- A. Gobbi, C. Scotti, G. Karnatzikos, A. Mudhigere, M. Castro, G.M. Peretti, One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years , Knee Surg. Sports Traumatol. Arthrosc. (2016) 1–8.
- A. Gobbi, G. Karnatzikos, C. Scotti, V. Mahajan, L. Mazzucco, B. Grigolo, One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-Year follow-up , Cartilage 2 (2011) 286–299.
- K.L. Wong, K.B.L. Lee, B.C. Tai, P. Law, E.H. Lee, J.H.P. Hui, Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up , Arthrosc. J. Arthrosc. Relat. Surg. 29 (2013) 2020–2028.
- J.M. Hare, J.E. Fishman, G. Gerstenblith, et al., Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the poseidon randomized trial, JAMA 308 (2012) 2369–2379.
- L. Wu, J.C.H. Leijten, N. Georgi, J.N. Post, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation , Tissue Eng. A 17 (2011) 1425–1436.
- L. Wu, H.-J. Prins, M.N. Helder, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells in chondrocyte Co-Cultures are independent of culture conditions and cell sources , Tissue Eng. A 18 (2012) 1542–1551.
- S.K. Sze, D.P.V. de Kleijn, R.C. Lai, E. Khia Way Tan, H. Zhao, K.S. Yeo, et al., Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells , Mol. Cell. Proteomics 6 (2007) 1680–1689.
- M.B. Murphy, K. Moncivais, A.I. Caplan, Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine , Exp. Mol. Med. 45 (2013) e54.
- M.J. Lee, J. Kim, M.Y. Kim, Y.-S. Bae, S.H. Ryu, T.G. Lee, et al., Proteomic analysis of tumor necrosis factor--induced secretome of human adipose tissue-derived mesenchymal stem cells , J. Proteome Res. 9 (2010) 1754–1762.
- S. Bruno, C. Grange, M.C. Deregibus, R.A. Calogero, S. Saviozzi, F. Collino, et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury, J. Am. Soc. Nephrol. 20 (2009) 1053–1067.
- M. Yá˜nez-Mó, P.R.-M. Siljander, Z. Andreu, A.B. Zavec, F.E. Borràs, E.I. Buzas, et al. Biological properties of extracellular vesicles and their physiological functions (2015).
- C. Lawson, J.M. Vicencio, D.M. Yellon, S.M. Davidson, Microvesicles and exosomes: new players in metabolic and cardiovascular disease , J. Endocrinol. 228 (2016) R57–R71.
- A.G. Thompson, E. Gray, S.M. Heman-Ackah, I. Mager, K. Talbot, S.E. Andaloussi, et al., Extracellular vesicles in neurodegenerative diseas—pathogenesis to biomarkers, Nat. Rev. Neurol. 12 (2016) 346–357.
- I.E.M. Bank, L. Timmers, C.M. Gijsberts, Y.-N. Zhang, A. Mosterd, J.-W. Wang, et al., The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease , Expert Rev. Mol. Diagn. 15 (2015) 1577–1588.
- T. Kato, S. Miyaki, H. Ishitobi, Y. Nakamura, T. Nakasa, M.K. Lotz, et al., Exosomes from IL-1 stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes , Arthritis. Res. Ther. 16 (2014) 1–11.
- R.W.Y. Yeo, S.K. Lim, Exosomes and their therapeutic applications, in: C. Gunther, A. Hauser, R. Huss (Eds.), Advances in Pharmaceutical Cell TherapyPrinciples of Cell-Based Biopharmaceuticals, World Scientific, Singapore, 2015, pp. 477–491.
- X. Qi, J. Zhang, H. Yuan, Z. Xu, Q. Li, X. Niu, et al., Exosomes secreted by human-Induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats , Int. J. Biol. Sci. 12 (2016) 836–849.
- R.C. Lai, F. Arslan, S.S. Tan, B. Tan, A. Choo, M.M. Lee, et al., Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles , J. Mol. Cell. Cardiol. 48 (2010) 1215–1224.
- Y. Zhou, H. Xu, W. Xu, B. Wang, H. Wu, Y. Tao, et al., Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro , Stem Cell Res. Ther. 4 (2013) 1–13.
- Y. Qin, L. Wang, Z. Gao, G. Chen, C. Zhang, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo , Sci. Rep. 6 (2016) 21961.
- M. Nakano, K. Nagaishi, N. Konari, Y. Saito, T. Chikenji, Y. Mizue, et al., Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes , Sci. Rep. 6 (2016) 24805.
- K. Nagaishi, Y. Mizue, T. Chikenji, M. Otani, M. Nakano, N. Konari, et al., Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes , Sci. Rep. 6 (2016) 34842.
- S.R. Baglio, K. Rooijers, D. Koppers-Lalic, F.J. Verweij, M. Pérez Lanzón, N. Zini, et al., Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species , Stem Cell Res. Ther. 6 (2015) 1–20.
- T. Chen, R. Yeo, F. Arslan, Y. Yin, S. Tan, Efficiency of exosome production correlates inversely with the developmental maturity of MSC donor, J. Stem Cell Res. Ther. 3 (2013) 2.
- R.C. Lai, S.S. Tan, B.J. Teh, S.K. Sze, F. Arslan, D.P. de Kleijn, et al., Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome , Int. J. Proteomics 2012 (2012) 971907.
- T.S. Chen, R.C. Lai, M.M. Lee, A.B.H. Choo, C.N. Lee, S.K. Lim, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs , Nucleic Acids Res. 38 (2010) 215–224.
- R.W. Yeo, R.C. Lai, K.H. Tan, S.K. Lim, Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell, J. Circ. Biomark. 1 (2013) 7.
- R.C. Lai, R.W. Yeo, S.K. Lim, Mesenchymal stem cell exosomes, Semin. Cell Dev. Biol. 40 (2015) 82–88.
- B. Zhang, R.W. Yeo, K.H. Tan, S.K. Lim, Focus on extracellular vesicles: therapeutic potential of stem cell-derived extracellular vesicles , Int. J. Mol. Sci. 17 (2016) 174.
- Hu G-w, Q. Li, X. Niu, B. Hu, J. Liu, Zhou S-m, et al., Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice , Stem Cell Res. Ther. 6 (2015) 1–15.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li, et al., Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis , J. Transl. Med. 13 (2015) 1–14.
- B. Zhang, M. Wang, A. Gong, X. Zhang, X. Wu, Y. Zhu, et al., HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing, Stem Cells 33 (2015) 2158–2168.
- B. Zhang, Y. Yin, R.C. Lai, S.S. Tan, A.B.H. Choo, S.K. Lim, Mesenchymal stem cells secrete immunologically active exosomes , Stem Cells Dev. 23 (2013) 1233–1244.
- C.Y. Tan, R.C. Lai, W. Wong, Y.Y. Dan, S.-K. Lim, H.K. Ho, Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models , Stem Cell Res. Ther. 5 (2014) 1–14.
- C. Lee, S.A. Mitsialis, M. Aslam, S.H. Vitali, E. Vergadi, G. Konstantinou, et al., Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension , Circulation 126 (2012) 2601–2611.
- B. Yu, H. Shao, C. Su, Y. Jiang, X. Chen, L. Bai, et al., Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1 , Sci. Rep. 6 (2016) 34562.
- Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof of concept clinical trial. Stem Cells. 2014;32(5):1254–66.
- Vega, Aurelio, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
- Davatchi F, Sadeghi-Abdollahi B, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–5
- Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case- controlled study. Int Orthop. 2014;38(9):1811–1818
- Galli D, Vitale M, Vaccarezza M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:6.
- Beitzel K, Solovyova O, Cote MP, et al. The future role of mesenchymal Stem cells in The management of shoulder disorders . Arthroscopy. 2013;29(10):1702–1711.
- Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190.
- Malda, Jos, et al. " Extracellular vesicles [mdash] new tool for joint repair and regeneration. " Nature Reviews Rheumatology (2016).
Further References about PRP
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4(1):52–62.
Extras
- Xu, Ming, et al. " Transplanted senescent cells induce an osteoarthritis-like condition in mice. " The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (2016): glw154.
- McCulloch, Kendal, Gary J. Litherland, and Taranjit Singh Rai. " Cellular senescence in osteoarthritis pathology ." Aging Cell (2017).
Patient Services at ANOVA Institute for Regenerative Medicine
- Located in the center of Germany, quick access by car or train from anywhere in Europe
- Simple access worldwide, less than 20 minutes from Frankfurt Airport
- Individualized therapy with state-of-the-art stem cell products
- Individually planned diagnostic work-up which include world-class MRI and CT scans
- German high quality standard on safety and quality assurance
- Personal service with friendly, dedicated Patient Care Managers
- Scientific collaborations with academic institutions to assure you the latest regenerative medical programs