BMC - Bone Marrow Stem Cell Therapy
ANOVA Institute for Regenerative Medicine - Offenbach, Germany
BMC is an innovative and minimally invasive therapeutic approach for one-time treatment of local problems, such as improving your orthopedic well-being and reducing pain from osteoarthritis, accelerating the healing of ligament and tendon injuries, tennis arm or elbow, problems of the hip, intervertebral discs or spine. Stem cell concentrate therapy is not to be confused with bone marrow transplantation, which is used for cancers such as blood cancers or lymphoma. Learn more about bone marrow concentrate and your personal treatment options at ANOVA Institue for Regenerative Medicine here.
Jump Directly to the Following Topics:
- What is BMC?
- Why is BMC a concentrate?
- Which disease are treatable with BMC?
- Workflow of a BMC Therapy at ANOVA
- Sources and literature
We Treat the Following Conditions With BMC:
- Osteoarthritis
- Knee - Meniscus Tears (medial and lateral), Chondromalacia Patella, Tendon Injuries (Patellar Tendonitis, Quad Tendon), Ligament sprains or tears (MCL, LCL, ACL)
- Hip - Hip Labrum Tears, SI Joint Dysfunction, Piriformis Syndrome, Greater Trochanteric Bursitis, Iliotibial Band (ITB) Syndrome
- Shoulder - Rotator Cuff Tendinitis, Tendonopathy, or Partial Tears, Labrum Tear, Bicipital Tendinitis
- Elbow - Lateral Epicondylitis (Tennis Elbow), Medial Epicondylitis (Golfers Elbow)
- Hand or Wrist Pain - DeQuervain's Tenosynovitis
- Ankle & Foot Pain - Achilles Tendinitis or Partial Tears, Plantar Fasciitis, Ankle sprains or ligament injury
- Spine - Facet Joint Arthropathy. Sacroiliac (SI) Joint Dysfunction
What is BMC - What is Bone Marrow Concentrate?
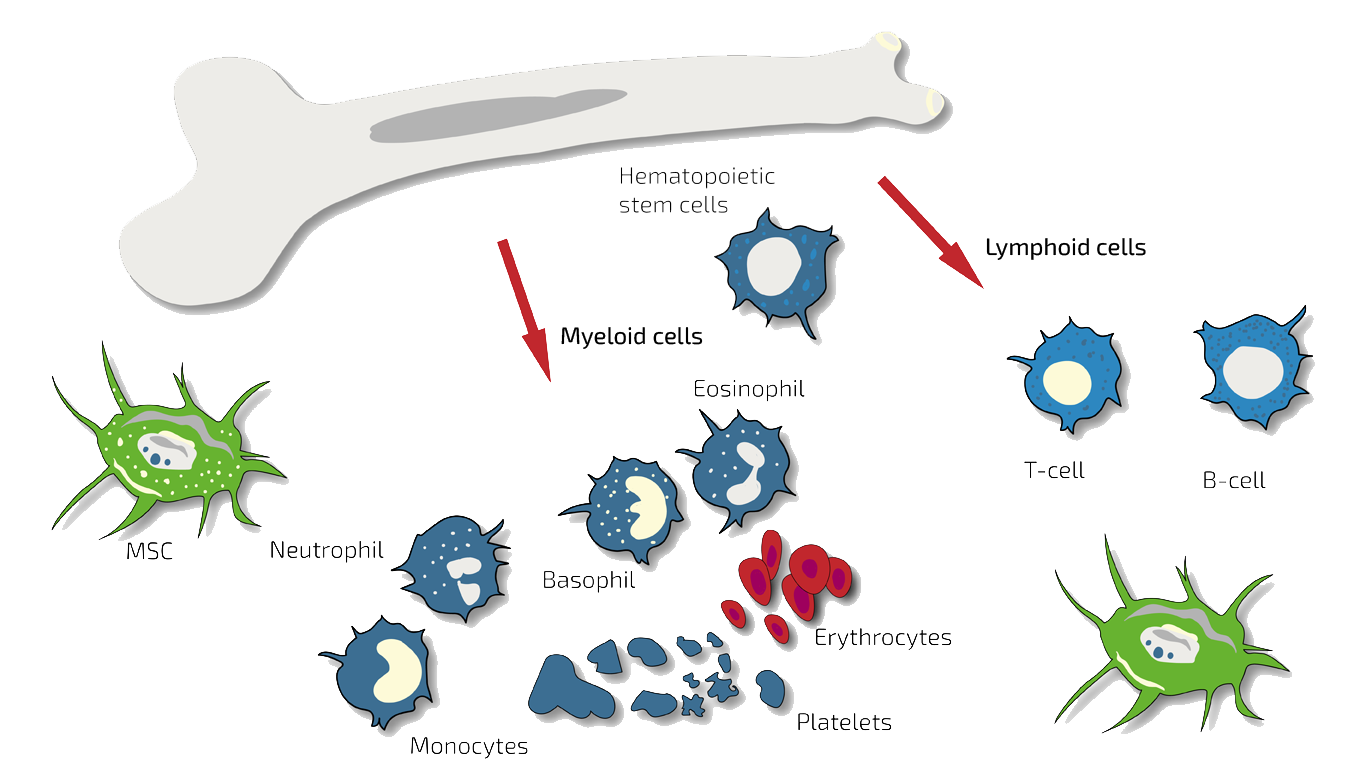
Bone Marrow Concentrate (BMC) is a fluid taken from the patient's iliac crest. It consists of blood and bone marrow stem cells, which are flushed out of the pelvic bone together when BMC is taken out using a syringe. The composition thus corresponds to the natural composition of a mixture of blood and bone marrow.
This mixture contains, among others, hematopoietic stem cells and so-called mesenchymal stem cells (named after their belonging to the middle layer of the body, the mesenchyme). In addition, the white blood cells found in the blood, such as myeloid cells (neutrophils, basophils, eosinophils, monocytes) and platelets, as well as lymphoid white blood cells (T cells and B cells).
Why is BMC a Stem Cell Concentrate?
BMC is named a stem cell concentrate because after the minimally invasive collection, the stem cells are concentrated to obtain as many stem cells as possible in little blood serum.
Blood serum and erythrocytes (red blood cells) are partially excluded from BMC, thereby increasing the stem cells concentration per mL.
This is especially important when the stem cells are to be injected into small joints because the joint space is very small. However, no further manipulation takes place on the BMCs.
The stem cells composition is the natural composition of bone marrow concentrated by approximately 6x.
The stem cells are not further manipulated, not cultivated or multiplied. They are re-applied directly after the finalization of all quality control tests.
The whole procedure of donation, concentration, quality control and re-application takes 1,5 hours.
Why Do We Get Stem Cells From the Bone Marrow?
Bone marrow can be found in the central region of your bones and is a soft spongy tissue which produces your red bloods cells, white blood cells and blood plasma components. Stem cells (hematopoietic as well as mesenchymal stem cells) can be found in this tissue, as they play a key role in the production of these cell components.
In contrast to other body regions that contain stem cells, the bone marrow is easily accessible and stem cells can be collected in a short, minimally invasive and safe procedure.
Stem cells are known to help advance healing processes and regeneration in tissue, and can be employed in moderate to severe osteoarthritis, tendon injuries and other orthopedic problems.
What Diseases Can Be Treated With BMC?
In general, the following diseases are treatable with BMC, meaning, they represent conditions for which it is probably that patients profit from stem cell treatment. However, we determine for each patient whether in this specific case stem cells are an advisable treatment strategy. Also there are several contraindications such as age under 18 years, pregnancy, breathing difficulties, acute cancer etc. Please inquire with our patient care managers whether BMC would be an option for you.
- Osteoarthritis
- Knee - Meniscus Tears (medial and lateral), Chondromalacia Patella, Tendon Injuries (Patellar Tendonitis, Quad Tendon), Ligament sprains or tears (MCL, LCL, ACL)
- Hip - Hip Labrum Tears, SI Joint Dysfunction, Piriformis Syndrome, Greater Trochanteric Bursitis, Iliotibial Band (ITB) Syndrome
- Shoulder - Rotator Cuff Tendinitis, Tendonopathy, or Partial Tears, Labrum Tear, Bicipital Tendinitis
- Elbow - Lateral Epicondylitis (Tennis Elbow), Medial Epicondylitis (Golfers Elbow)
- Hand or Wrist Pain - DeQuervain's Tenosynovitis
- Ankle & Foot Pain - Achilles Tendinitis or Partial Tears, Plantar Fasciitis, Ankle sprains or ligament injury
- Spine - Facet Joint Arthropathy. Sacroiliac (SI) Joint Dysfunction
More medical applications may be possible, please consult our doctors.
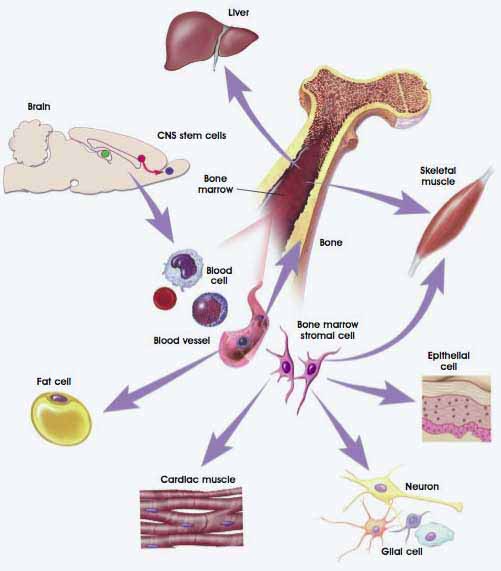
Figure 1: Plasticity Among Adult Stem Cells. (© 2001 Terese Winslow, Lydia Kibiuk, Caitlin Duckwall)
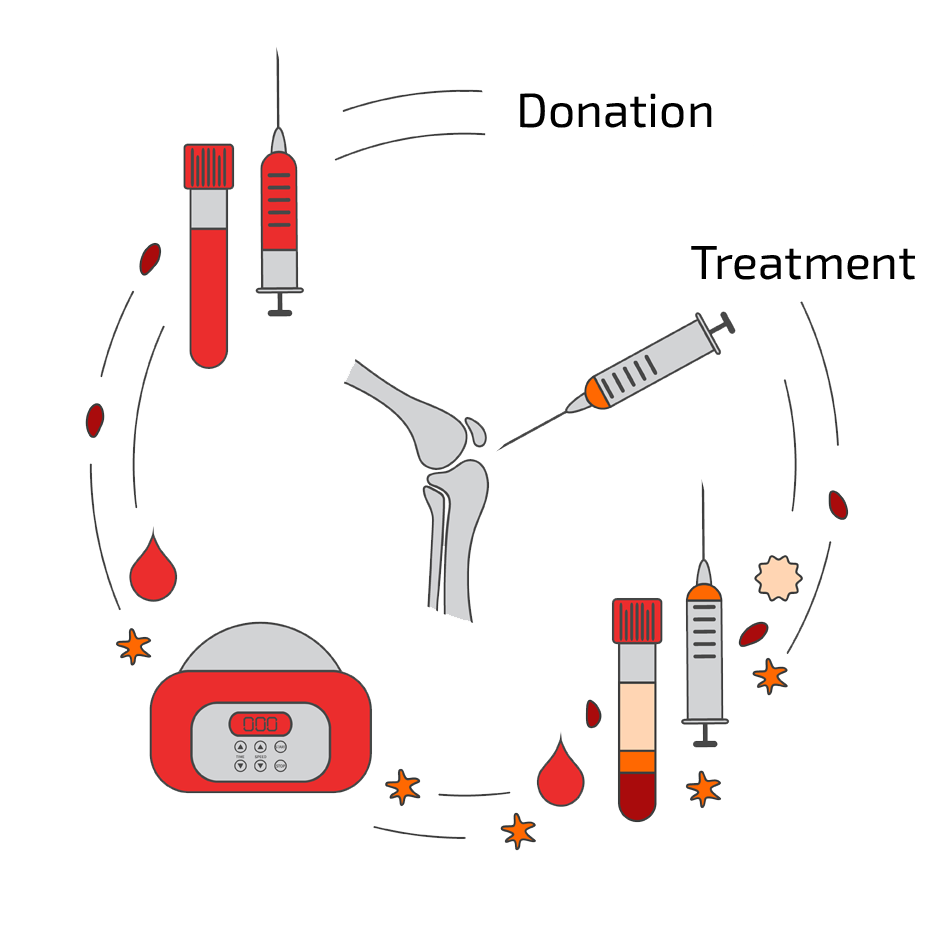
Workflow of a BMC Therapy
In the schema below we have summarized the steps towards your individual BMC therapy form initial free-of-charge consultation with our patient care managers up the the final BMC application in Offenbach, Germany.
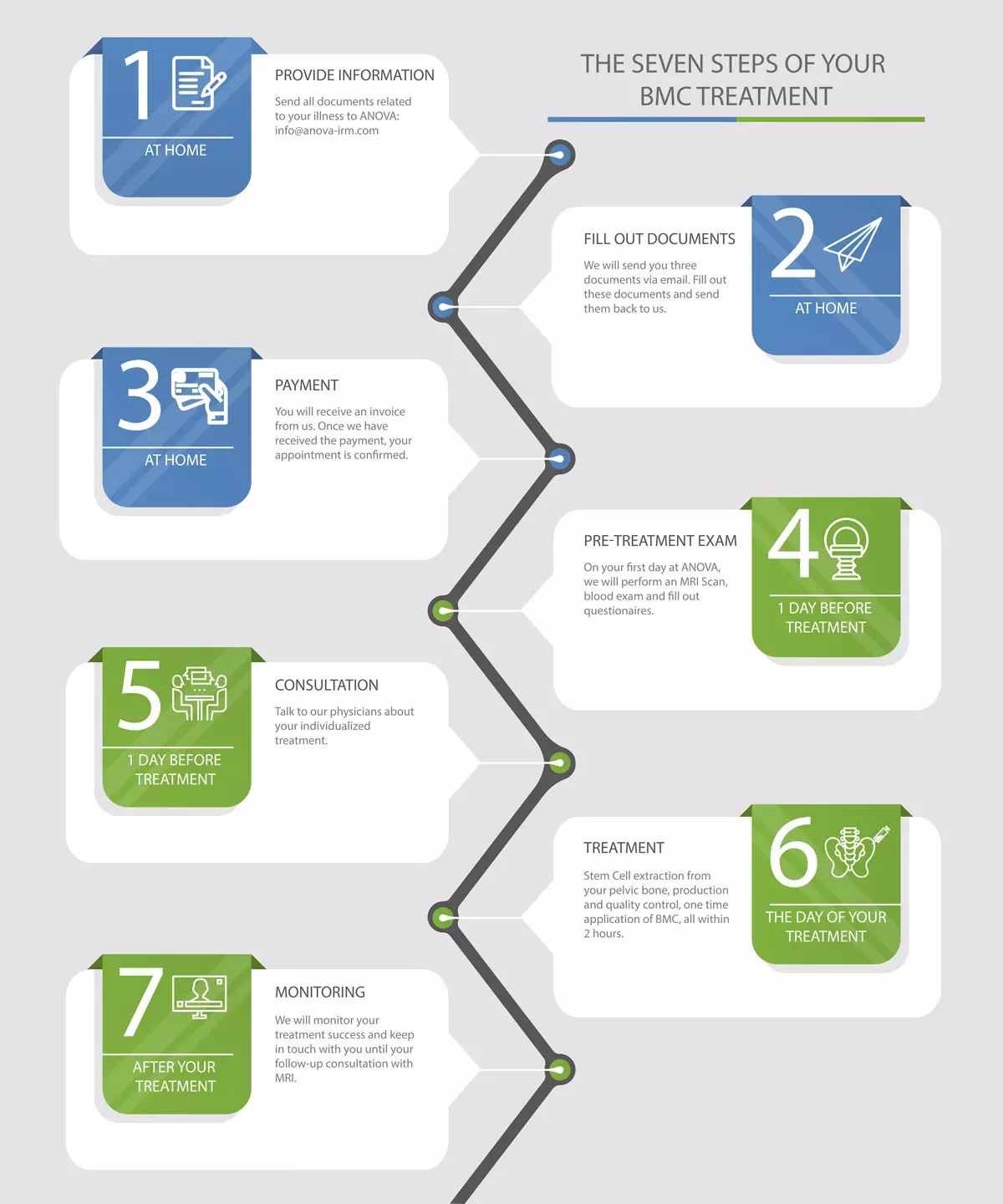
The 7 steps of a BMC Therapy
ANOVA – Leading Expert in the Field of BMC Application
ANOVA, a German clinic for regenerative medicine, is one of the first licensed institutions for BMC in Europe. ANOVA has employed BMC as a non-surgical regenerative treatment for different applications, with focus in orthopedics, since 2011. Talk to our experienced and knowledgeable physicians today, to learn more about your treatment options at ANOVA.
ANOVA has acquired two pharmaceutical manufacturing licenses for stem cell products in 2018 and is therefore, a continuously authority-controlled clinic. This guarantees you safe, controlled and reliable products.
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
How BMC Therapy Works
Bone Marrow Concentrate (BMC) has the ability to utilize the body’s own ability to regenerate and heal itself through the containing growth factors and regenerative cells. BMC contains pluripotent cells, which means that they are able to turn themselves into various different types of tissue. The detailed mechanisms of stem cell action are not well understood but somehow stem cells are able to stop over-flowing immune reactions thereby reducing or stopping damaging inflammation and thereafter, they are able to stimulate regeneration.
While some types of stem cells have failed to show healing effects in conditions such as stroke and heart diseases, bone marrow stem cells have been proven to be effective for a number of other applications. The data is available at the prestigious Cochrane Library, the international authority for evidence-based medicine.
Interestingly, the previously ignored blood cells of the bone marrow appear to play a pivotal role in many healing processes. They contain high numbers of cells with CD34+ and c-kit surface markers, without which stem cells are ineffective in repairing and regenerating damaged tissues and organs.
The Story of BMC Therapy
Early research in stem cells has focused on investigating the regenerative capabilities of embryonic or adult Mesenchymal Stem Cells. While scientists and doctors still hope they can use these cells for the treatment of many diseases, they cannot be used for treating patients in most countries, due to legal restrictions and safety concerns.
Therefore, doctors and scientists began searching for alternatives to employ this cutting edge approach. Bone marrow stem cells are an obvious alternative. They are abundant, relatively easy to access and both the harvest as well as the application has minimal potential of health risks or side effects.
For many years, critics of stem cell treatments claimed that bone marrow stem cells are not effective, because they mainly contain blood forming cells and only a low number of Mesenchymal Stem Cells (MSCs). In short, the critics claimed that bone marrow stem cell treatment is only good for making money, but not for curing patients. Scientific evidence, however, has shown significant effects of Bone Marrow Cells.
Stem Cell Therapies sorted by stem cell type (source tissue) and product
References and Literature - Bone Marrow Concentrate 'BMC'
- 1. He Y, He W, Qin G, Luo J, Xiao M. Transplantation KCNMA1 modified bone marrow-mesenchymal stem cell therapy for diabetes mellitus-induced erectile dysfunction. Andrologia. 2014;46(5):479-486. doi:10.1111/and.12104.
- Mathiasen AB, Qayyum AA, Jørgensen E, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial ({MSC}-{HF} trial). Eur Heart J. 2015;36(27):1744-1753. doi:10.1093/eurheartj/ehv136.
- Mathiasen AB, Qayyum AA, Jørgensen E, et al. Interventional cardiology Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure : a randomized placebo-controlled trial. 2015. doi:10.1093/eurheartj/ehv136.
- Liao H-T, Chen C-T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6(3):288-295. doi:10.4252/wjsc.v6.i3.288.
- Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24(10):2292-2298. doi:10.1634/stemcells.2005-0542.
- 2015_Cao_Spine-Journal_Bone-marrow-mesenchymal-stem-cells-slow-intervertebral-disc-degeneration-through-the-NF-κB-pathway.pdf.
- Zhao J, Zhang Q, Wang Y, Li Y. Uterine Infusion With Bone Marrow Mesenchymal Stem Cells Improves Endometrium Thickness in a Rat Model of Thin Endometrium. Reprod Sci. 2015;22(2):181-188. doi:10.1177/1933719114537715.
- Fekete N, Rojewski MT, Fürst D, et al. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 2012;7(8). doi:10.1371/journal.pone.0043255.
- Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. 2014:4-11.
- Li C, Wu X, Tong J, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6(1):55. doi:10.1186/s13287-015-0066-5.
- Books J, Sign R. Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postrad ... Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postradical Prostatectomy Erectile Dysfunction : An Open Dose-Escalation Pilot Study Safety of Intracavernous Bone Marrow-Mon. 2017:2015-2017.
- Rinker TE, Hammoudi TM, Kemp ML, Lu H, Temenoff JS. Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment. Integr Biol (Camb). 2014;6(3):324-337. doi:10.1039/c3ib40194d.
- Al-sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. 2015:269-276.
- Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016;51(6):942-947. doi:10.1016/j.jpedsurg.2016.02.061.
- Tate-oliver K, Alexander RW. Density Platelet-Rich Plasma or Bone Marrow.
- Tang K, Yan J, Shen Y, et al. Tracing type 1 diabetic Tibet miniature pig ’ s bone marrow mesenchymal stem cells in vitro by magnetic resonance imaging. 2014;6:123-131. doi:10.1111/1753-0407.12084.
- Wang X, Mamillapalli R, Mutlu L, Du H, Taylor HS. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15(1):14-22. doi:10.1016/j.scr.2015.04.004.
- Rambaldi A, Capelli C, Domenghini M, et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. 2007:785-791. doi:10.1038/sj.bmt.1705798.
- Mushtaq M, Williams AR, Suncion VY, et al. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy The TAC-HFT Randomized Trial. 2015;16960. doi:10.1001/jama.2013.282909.
- 2014 Autografting of bone marrow mesenchymal stem cells alleviates streptozotocin induced diabetes in miniature pigs.pdf.
- Program T, Marga- P, Kingdom U. Bone Marrow Therapies for Chronic Heart Dis- ease. 2015:1-12. doi:10.1002/stem.2080.
- Biology C, Cell R, Eye N, Institutes N, Infor- AS. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 2017:1273-1285. doi:10.1002/sctm.12056.
- Jeong Y, Kyu H, Hwa H, Chan Y. Cellular Physiology and Biochemistr y Biochemistry Direct Comparison of Human Mesenchymal Stem Cells Derived from Adipose Tissues and Bone Marrow in Mediating Neovascularization in Response to Vascular Ischemia. Cell Physiol Biochem. 2007;20:867-876.
- Shutian S, Shaoping N, Xingxin W, et al. GW25-e3198 The combination of transforming growth factor β1 and 5-azacytidine improve the differentiation effects of rat Bone marrow mesenchymal stem cells into cardiomyocytes. J Am Coll Cardiol. 2014;64(16):C21. doi:10.1016/j.jacc.2014.06.105.
- Narita T, Suzuki K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail Rev. 2014:53-68. doi:10.1007/s10741-014-9435-x.
- Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond). 2014;11:1. doi:10.1186/1476-9255-11-1.
- Dong X, Zhu F, Liu Q, et al. Transplanted bone marrow mesenchymal stem cells protects myocardium by regulating 14-3-3 protein in a rat model of diabetic cardiomyopathy. 2014;7(7):3714-3723.
- Huang L, Wu W, Luo F. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes : A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. 2016:1-9. doi:10.2337/dc15-0171.
- Sanghi V, Sethi D, Harris KL, et al. International Journal of the Cardiovascular Academy Autologous bone marrow concentrate enriched in progenitor cells — An adjuvant in the treatment of acute myocardial infarction. IJCAC. 2016. doi:10.1016/j.ijcac.2016.04.001.
- Leyh M, Seitz A, Dürselen L, et al. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. 2014:1-18. doi:10.1186/s13075-014-0453-9.
- Surgery M, Stomatological S, Material CP, et al. T ISSUE -S PECIFIC S TEM C ELLS Adiponectin Regulates Bone Marrow Mesenchymal Stem Cell Niche Through a Unique Signal Transduction Pathway : An Approach for Treating Bone Disease in Diabetes. 2015:240-252.
- Li C, Wu X, Tong J, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6(1):55. doi:10.1186/s13287-015-0066-5.
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-Mediated Neuroprotection and Neuritogenesis of Axotomised Retinal Ganglion Cells by Human Dental Pulp Stem Cells: Comparison with Human Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. PLoS One. 2014;9(10):e109305. doi:10.1371/journal.pone.0109305.
- Cao C, Zou J, Liu X, Shapiro A. Bone marrow mesenchymal stem cells slow intervertebral disc degeneration through the NF- k B pathway. Spine J. 2015;15(3):530-538. doi:10.1016/j.spinee.2014.11.021.
- Li C, Wu X, Tong J, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. ??? 2015. doi:10.1186/s13287-015-0066-5.
- Narita T, Suzuki K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. 2015:53-68. doi:10.1007/s10741-014-9435-x.
- Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy. Jama. 2014;311(1):62. doi:10.1001/jama.2013.282909.
- Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. doi:10.1186/s13287-015-0116-z.
- Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. doi:10.1038/nm.2736.
- Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. doi:10.1186/s13287-015-0116-z.
- Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92(4):387-397. doi:10.1007/s00109-013-1110-5.
- Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888-H1897. doi:10.1152/ajpheart.00186.2009.
- Czubak PB, Bojarska-junak A, Tabarkiewicz J, Putowski LB. A Modified Method of Insulin Producing Cells ’ Generation from Bone Marrow-Derived Mesenchymal Stem Cells. 2014;2014:1-7. doi:10.1155/2014/628591.
- Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. doi:10.1186/s13287-015-0116-z.
- am Esch JS, Knoefel WT, Klein M, et al. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;23(4):463-470. doi:10.1634/stemcells.2004-0283.
- Wang X, Nie S-P, Zhen L, et al. TCTAP A-156 Retrograde Coronary Vein Delivery of Basic Fibroblast Growth Enhances Bone Marrow Mesenchymal Stem Cells Engraftment for Myocardial Repair in a Canine Infarct Model. J Am Coll Cardiol. 2014;63(12):S44. doi:10.1016/j.jacc.2014.02.189.
- Al-sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. 2015:269-276.
- Gabr MM, Zakaria MM, Refaie AF, et al. Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells into Insulin-Producing Cells : Evidence for Further Maturation In Vivo. 2015;2015.
- Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103(8):1952-1958. doi:10.1111/j.1572-0241.2008.01993.x.
- Kushida T, Iida H. Bone marrow cell transplantation efficiently repairs tendon and ligament injuries. Front Cell Dev Biol. 2014;2(July):1-4. doi:10.3389/fcell.2014.00027.
- Schulte J, Knoefel T, Klein M, et al. Portal Application of Autologous CD133 + Bone Marrow Cells to the. 2005:463-470. doi:10.1634/stemcells.2004-0283.
- Scott M, Ph D, Marley SB, et al. Autologous Infusion of Expanded Mobilized Adult Bone Marrow-Derived CD34 + Cells Into Patients With Alcoholic Liver Cirrhosis. 2008:1952-1958. doi:10.1111/j.1572-0241.2008.01993.x.
- 2015 Murine Sca1+Lin− bone marrow contains an endodermal precursor population that differentiates into hepatocytes.pdf.
- Prabhakar S, Marwaha N, Lal V, Sharma RR, Rajan R, Khandelwal N. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: A pilot study. Neurol India. 2012;60(5):465-469. doi:10.4103/0028-3886.103185.
- Naaldijk Y, Jäger C, Fabian C, et al. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2016:1-16. doi:10.1111/nan.12319.
- Nicola M Di, Carlo-stella C, Magni M, et al. induced by cellular or nonspecific mitogenic stimuli Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. 2013;99(10):3838-3843. doi:10.1182/blood.V99.10.3838.
- Tzameret A, Sher I, Belkin M, et al. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration. Stem Cell Res. 2015;15(2):387-394. doi:10.1016/j.scr.2015.08.007.
- Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175-1186. doi:10.1002/art.22511.
- Capelli C, Domenghini M, Borleri G, et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant. 2007;40(8):785-791. doi:10.1038/sj.bmt.1705798.
- Sanghi V, Sethi D, Harris KL, et al. International Journal of the Cardiovascular Academy Autologous bone marrow concentrate enriched in progenitor cells — An adjuvant in the treatment of acute myocardial infarction. IJCAC. 2016. doi:10.1016/j.ijcac.2016.04.001.
- Oe K, Kushida T, Okamoto N, et al. New strategies for anterior cruciate ligament partial rupture using bone marrow transplantation in rats. Stem Cells Dev. 2011;20(4):671-679. doi:10.1089/scd.2010.0182.
- Ahmed HH, Salem AM, Atta HM, et al. Updates in the pathophysiological mechanisms of Parkinson’s disease: Emerging role of bone marrow mesenchymal stem cells. World J Stem Cells. 2016;8(3):106. doi:10.4252/wjsc.v8.i3.106.
- Cai J, Wu Z, Xu X, et al. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care. 2015:dc150171. doi:10.2337/dc15-0171.
- Abdel Aziz MT, Wassef MAA, Ahmed HH, et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr. 2014;6(1):34. doi:10.1186/1758-5996-6-34.
- Al-sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. 2015:269-276.
- Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888-H1897. doi:10.1152/ajpheart.00186.2009.
- Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7). doi:10.1371/journal.pone.0011803.
- Sanghi V, Sethi D, Harris KL, et al. International Journal of the Cardiovascular Academy Autologous bone marrow concentrate enriched in progenitor cells — An adjuvant in the treatment of acute myocardial infarction. IJCAC. 2016. doi:10.1016/j.ijcac.2016.04.001.
- Fekete N, Rojewski MT, Fürst D, et al. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 2012;7(8). doi:10.1371/journal.pone.0043255.
- C. L, S.A. M, M. A, S.H. V, A. F-G. Exosomes mediate the cytoprotective effects of bone Marrow-Derived Stromal Cells (MSCS) on the hypoxic lung. Am J Respir Crit Care Med. 2011;183(1 MeetingAbstracts):no pagination. http://ajrccm.atsjournals.org/cgi/reprint/183/1_MeetingAbstracts/A3764?sid=f0b58cd0-9f08-401b-bb88-e9a3268d044f%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70848115.
- Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. doi:10.1038/nm.2736.
- Yoon SH, Shim YS, Park YH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25(8):2066-2073. doi:10.1634/stemcells.2006-0807.
- Hernigou P, Guissou I, Homma Y, et al. Percutaneous injection of bone marrow mesenchymal stem cells for ankle non-unions decreases complications in patients with diabetes. Int Orthop. 2015. doi:10.1007/s00264-015-2738-2.
- Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA. Cryobiology The effects of cryopreservation on cells isolated from adipose , bone marrow and dental pulp tissues q. Cryobiology. 2014;69(2):342-347. doi:10.1016/j.cryobiol.2014.08.003.
- C. L, S.A. M, M. A, S.H. V, A. F-G. Exosomes mediate the cytoprotective effects of bone Marrow-Derived Stromal Cells (MSCS) on the hypoxic lung. Am J Respir Crit Care Med. 2011;183(1 MeetingAbstracts):no pagination. http://ajrccm.atsjournals.org/cgi/reprint/183/1_MeetingAbstracts/A3764?sid=f0b58cd0-9f08-401b-bb88-e9a3268d044f%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70848115.
- Tang KX, Shen YF, Li BY, et al. Tracing type 1 diabetic Tibet miniature pig’s bone marrow mesenchymal stem cells in vitro by magnetic resonance imaging. J Diabetes. 2013;6:123-131. doi:10.1111/1753-0407.12084.
- Associates RM, Biosciences C. T RANSLATIONAL AND C LINICAL Percutaneous Injection of Autologous Bone Marrow Concentrate Cells Significantly Reduces Lumbar Discogenic Pain Through 12 Months. 2015:146-156.
- Kasahara Y, Matsuyama T, Taguchi A. Treatment of Autologous Bone Marrow Mononuclear Cells for Acute and Subacute Stroke Cell Therapy for Acute / Subacute Stroke. 2015:37-46. doi:10.1007/978.
- Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12(7):836-849. doi:10.7150/ijbs.14809.
- Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888-H1897. doi:10.1152/ajpheart.00186.2009.
- Nicola M Di, Carlo-stella C, Magni M, et al. induced by cellular or nonspecific mitogenic stimuli Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. 2013;99(10):3838-3843. doi:10.1182/blood.V99.10.3838.
- Capelli C, Domenghini M, Borleri G, et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant. 2007;40(8):785-791. doi:10.1038/sj.bmt.1705798.
- Yiou R, Hamidou L, Birebent B, et al. Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postradical Prostatectomy Erectile Dysfunction: An Open Dose-Escalation Pilot Study. Eur Urol. 2016;69(6):988-991. doi:10.1016/j.eururo.2015.09.026.
- Neural I, Medicine R, Science M. Complete Spinal Cord Injury Treatment Using Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation with Granulocyte Macrophage-Colony Stimulating Factor : Phase I / II Clinical Trial. 2007:2066-2073. doi:10.1634/stemcells.2006-0807.
- Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7). doi:10.1371/journal.pone.0011803.
- Yu L, Tu Q, Han Q, et al. Adiponectin Regulates Bone Marrow Mesenchymal Stem Cell Niche Through a Unique Signal Transduction Pathway : An Approach for Treating Bone Disease in Diabetes. Stem Cells. 2015;33:240-252. doi:10.1002/stem.1844.
- Lyra AC, Soares MB, da Silva LF, et al. Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol. 2007;13(7):1067-1073. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17373741.
- Song F, Tang J, Geng R, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. 2014;7(4):1415-1426.
- Nakamura-ishizu A, Takubo K, Kobayashi H, Suzuki-inoue K. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. 2015. doi:10.1084/jem.20150057.
- Leyh M, Seitz A, Dürselen L, et al. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. 2014:1-18. doi:10.1186/s13075-014-0453-9.
- Beane OS, Fonseca VC, Cooper LL, Koren G. Impact of Aging on the Regenerative Properties of Bone Marrow- , Muscle- , and Adipose-Derived Mesenchymal Stem / Stromal Cells. 2014:1-22. doi:10.1371/journal.pone.0115963.
- Nicola M Di, Carlo-stella C, Magni M, et al. induced by cellular or nonspecific mitogenic stimuli Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. 2013;99(10):3838-3843. doi:10.1182/blood.V99.10.3838.
- Ribeiro A, Laranjeira P, Mendes S, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4(5):125. doi:10.1186/scrt336.
- Observer C, Alto P, Program T, Hospital M. T RANSLATIONAL AND C LINICAL Bone Marrow Therapies for Chronic Heart Disease. 2015:3212-3227. doi:10.1002/stem.2080.
- Beane OS, Fonseca VC, Cooper LL, Koren G. Impact of Aging on the Regenerative Properties of Bone Marrow- , Muscle- , and Adipose-Derived Mesenchymal Stem / Stromal Cells. 2014:1-22. doi:10.1371/journal.pone.0115963.
- Rodriguez-Menocal L, Shareef S, Salgado M, Shabbir A, Van Badiavas E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res Ther. 2015;6(1):1-11. doi:10.1186/s13287-015-0001-9.
- Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. doi:10.1038/nm.2736.
- Venkataramana NK, Kumar SK V, Balaraju S, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155(2):62-70. doi:10.1016/j.trsl.2009.07.006.
- Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells’ generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014;2014:628591. doi:10.1155/2014/628591.
- Shutian S, Shaoping N, Xingxin W, et al. GW25-e3198 The combination of transforming growth factor β1 and 5-azacytidine improve the differentiation effects of rat Bone marrow mesenchymal stem cells into cardiomyocytes. J Am Coll Cardiol. 2014;64(16):C21. doi:10.1016/j.jacc.2014.06.105.
- Yang J, Kaur K, Ong LL, Eisenberg CA, Eisenberg LM. Inhibition of G9a Histone Methyltransferase Converts Bone Marrow Mesenchymal Stem Cells to Cardiac Competent Progenitors. Stem Cells Int. 2015;2015:1-12. doi:10.1155/2015/270428.
- Biology C, Cell R, Eye N, Institutes N, Infor- AS. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 2017. doi:10.1002/sctm.12056.
- Chen J, Venkat P, Chopp M. Bone Marrow Mesenchymal Stromal Cell Transplantation : A Neurorestorative Therapy for Stroke. 2015:47-69. doi:10.1007/978.
- Kondo M, Kamiya H, Himeno T, et al. Therapeutic efficacy of bone marrow-derived mononuclear cells in diabetic polyneuropathy is impaired with aging or diabetes. J Diabetes Investig. 2015;6(2):140-149. doi:10.1111/jdi.12272.
- Nakamura-ishizu A, Takubo K, Kobayashi H, Suzuki-inoue K. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. 2015. doi:10.1084/jem.20150057.
- Gabr MM, Zakaria MM, Refaie AF, et al. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed Res Int. 2014;2014:832736. doi:10.1155/2014/832736.
- Tzameret A, Sher I, Belkin M, et al. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration ☆. Stem Cell Res. 2015;15(2):387-394. doi:10.1016/j.scr.2015.08.007.
- C. L, S.A. M, M. A, S.H. V, A. F-G. Exosomes mediate the cytoprotective effects of bone Marrow-Derived Stromal Cells (MSCS) on the hypoxic lung. Am J Respir Crit Care Med. 2011;183(1 MeetingAbstracts):no pagination. http://ajrccm.atsjournals.org/cgi/reprint/183/1_MeetingAbstracts/A3764?sid=f0b58cd0-9f08-401b-bb88-e9a3268d044f%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70848115.
- Sun J, Wei ZZ, Gu X, et al. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol. 2015. doi:10.1016/j.expneurol.2015.03.011.
- Talaat M, Aziz A, Abdel M, et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. 2014:1-10.
- Song F, Tang J, Geng R, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. 2014;7(4):1415-1426.
- Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease. Gut. 2007;56(5):716-724. doi:10.1136/gut.2006.098442.
- Kondo M, Kamiya H, Himeno T, et al. Therapeutic ef fi cacy of bone marrow-derived mononuclear cells in diabetic polyneuropathy is impaired with aging or diabetes. 2015;6(2). doi:10.1111/jdi.12272.
- Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells’ generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014;2014:628591. doi:10.1155/2014/628591.
- Beane OS, Fonseca VC, Cooper LL, Koren G. Impact of Aging on the Regenerative Properties of Bone Marrow- , Muscle- , and Adipose-Derived Mesenchymal Stem / Stromal Cells. 2014:1-22. doi:10.1371/journal.pone.0115963.
- Song F, Tang J, Geng R, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. 2014;7(4):1415-1426.
- Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA. Cryobiology The effects of cryopreservation on cells isolated from adipose , bone marrow and dental pulp tissues q. Cryobiology. 2014;69(2):342-347. doi:10.1016/j.cryobiol.2014.08.003.
- Sanz-Ruiz R, Garcia AN, Elizaga J, Fdez-Aviles F. TCT-150 First-in-man Experience with Transendocardial Injections of Bone Marrow-Derived Mesenchymal Stem Cells in Idiopathic Dilated Cardiomyopathy. The MYOCYTE trial. J Am Coll Cardiol. 2014;64(11):B45. doi:10.1016/j.jacc.2014.07.186.
- Totey SM. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinsons disease. Transl Res. 2010;155(2):62-70. doi:10.1016/j.trsl.2009.07.006.
- Biology C, Cell R, Eye N, Institutes N. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 2017:1273-1285.
- Biology C, Cell R, Eye N, Institutes N. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 2017.
- Naaldijk Y, J C. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP / PS1 Alzheimer mice. 2016:1-16. doi:10.1111/nan.12319.
- Development CD. C ELL -B ASED D RUG D EVELOPMENT , S CREENING , AND T OXICOLOGY Phase I Trial of Repeated Intrathecal Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in Amyotrophic Lateral Sclerosis. 2015:590-597.
- Prabhakar S, Marwaha N, Lal V, Sharma RR, Rajan R, Khandelwal N. amyotrophic lateral sclerosis : A pilot study Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis : A pilot study. 2016;(September 2012). doi:10.4103/0028-3886.103185.
Further References for MSC, BMC, Stemcell Secretome and EVs
- Georg Hansmann, Philippe Chouvarine, Franziska Diekmann, Martin Giera, Markus Ralser, Michael Mülleder, Constantin von Kaisenberg, Harald Bertram, Ekaterina Legchenko & Ralf Hass "Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension". Nature Cardiovascular Research volume 1, pages568–576 (2022).
- Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis . Arthritis Rheum. 2003;48:3464–74.
- Lee KB, Hui JH, Song IC, Ardany L, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cell. 2007;25:2964–71.
- Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy . 2009;25(12):1391–400.
- Black L, Gaynor J, Adams C, et al. Effect of intra-articular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200.
- Centeno C, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–53.
- Centeno C, Kisiday J, Freeman M, et al. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: a case study. Pain Physician. 2006;9:253–6.
- Centeno C, Schultz J, Cheever M. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell. 2011;5(1):81–93.
- Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose derived stem cells: a case series. J Med Case Rep. 2001;5:296.
- Kuroda R, Ishida K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31.
- Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422–8.
- Saw KY et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94.
- Vangsness CT, Farr J, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg. 2014;96(2):90–8.
- Freitag, Julien, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy–a review. BMC musculoskeletal disorders 17.1 (2016): 230.
- Maumus, Marie, Christian Jorgensen, and Danièle Noël. " Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. " Biochimie 95.12 (2013): 2229-2234.
- Dostert, Gabriel, et al. " How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication?. " Frontiers in Cell and Developmental Biology 5 (2017).
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- Toh, Wei Seong, et al. " MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. " Seminars in Cell & Developmental Biology. Academic Press, 2016.
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- S. Koelling, J. Kruegel, M. Irmer, J.R. Path, B. Sadowski, X. Miro, et al., Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis , Cell Stem Cell 4 (2009) 324–335.
- B.A. Jones, M. Pei, Synovium-Derived stem cells: a tissue-Specific stem cell for cartilage engineering and regeneration , Tissue Eng. B: Rev. 18 (2012) 301–311.
- W. Ando, J.J. Kutcher, R. Krawetz, A. Sen, N. Nakamura, C.B. Frank, et al., Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage, Cytotherapy 16 (2014) 776–788.
- K.B.L. Lee, J.H.P. Hui, I.C. Song, L. Ardany, E.H. Lee, Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model, Stem Cells 25 (2007) 2964–2971.
- W.-L. Fu, C.-Y. Zhou, J.-K. Yu, A new source of mesenchymal stem cells for articular cartilage repair: mSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model , Am. J. Sports Med. 42 (2014) 592–601.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, et al., Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration , Biomaterials 33 (2012) 7008–7018.
- E.-R. Chiang, H.-L. Ma, J.-P. Wang, C.-L. Liu, T.-H. Chen, S.-C. Hung, Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits , PLoS One 11 (2016) e0149835.
- H. Nejadnik, J.H. Hui, E.P. Feng Choong, B.-C. Tai, E.H. Lee, Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study , Am. J. Sports Med. 38 (2010) 1110–1116.
- I. Sekiya, T. Muneta, M. Horie, H. Koga, Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects , Clin. Orthop. Rel. Res. 473 (2015) 2316–2326.
- Y.S. Kim, Y.J. Choi, Y.G. Koh, Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes , Am. J. Sports Med. 43 (2015) 2293–2301.
- W.-L. Fu, Y.-F. Ao, X.-Y. Ke, Z.-Z. Zheng, X. Gong, D. Jiang, et al., Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment , Knee 21 (2014) 609–612.
- Y.-G. Koh, O.-R. Kwon, Y.-S. Kim, Y.-J. Choi, D.-H. Tak, Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial , Arthrosc. J. Arthrosc. Relat. Surg. 32 (2016) 97–109.
- T.S. de Windt, L.A. Vonk, I.C.M. Slaper-Cortenbach, M.P.H. van den Broek, R. Nizak, M.H.P. van Rijen, et al., Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-Stage cartilage repair in humans upon mixture with recycled autologous chondrons , Stem Cells (2016) (n/a-n/a).
- L. da Silva Meirelles, A.M. Fontes, D.T. Covas, A.I. Caplan, Mechanisms involved in the therapeutic properties of mesenchymal stem cells , Cytokine Growth Factor Rev. 20 (2009) 419–427.
- W.S. Toh, C.B. Foldager, M. Pei, J.H.P. Hui, Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration , Stem Cell Rev. Rep. 10 (2014) 686–696.
- R.C. Lai, F. Arslan, M.M. Lee, N.S.K. Sze, A. Choo, T.S. Chen, et al., Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury , Stem Cell Res. 4 (2010) 214–222.
- S. Zhang, W.C. Chu, R.C. Lai, S.K. Lim, J.H.P. Hui, W.S. Toh, Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration, Osteoarthr . Cartil. 24 (2016) 2135–2140.
- S. Zhang, W. Chu, R. Lai, J. Hui, E. Lee, S. Lim, et al., 21 – human mesenchymal stem cell-derived exosomes promote orderly cartilage regeneration in an immunocompetent rat osteochondral defect model , Cytotherapy 18 (2016) S13.
- C.T. Lim, X. Ren, M.H. Afizah, S. Tarigan-Panjaitan, Z. Yang, Y. Wu, et al., Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model
- A. Gobbi, G. Karnatzikos, S.R. Sankineani, One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee , Am. J. Sports Med. 42 (2014) 648–657.
- A. Gobbi, C. Scotti, G. Karnatzikos, A. Mudhigere, M. Castro, G.M. Peretti, One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years , Knee Surg. Sports Traumatol. Arthrosc. (2016) 1–8.
- A. Gobbi, G. Karnatzikos, C. Scotti, V. Mahajan, L. Mazzucco, B. Grigolo, One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-Year follow-up , Cartilage 2 (2011) 286–299.
- K.L. Wong, K.B.L. Lee, B.C. Tai, P. Law, E.H. Lee, J.H.P. Hui, Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up , Arthrosc. J. Arthrosc. Relat. Surg. 29 (2013) 2020–2028.
- J.M. Hare, J.E. Fishman, G. Gerstenblith, et al., Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the poseidon randomized trial, JAMA 308 (2012) 2369–2379.
- L. Wu, J.C.H. Leijten, N. Georgi, J.N. Post, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation , Tissue Eng. A 17 (2011) 1425–1436.
- L. Wu, H.-J. Prins, M.N. Helder, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells in chondrocyte Co-Cultures are independent of culture conditions and cell sources , Tissue Eng. A 18 (2012) 1542–1551.
- S.K. Sze, D.P.V. de Kleijn, R.C. Lai, E. Khia Way Tan, H. Zhao, K.S. Yeo, et al., Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells , Mol. Cell. Proteomics 6 (2007) 1680–1689.
- M.B. Murphy, K. Moncivais, A.I. Caplan, Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine , Exp. Mol. Med. 45 (2013) e54.
- M.J. Lee, J. Kim, M.Y. Kim, Y.-S. Bae, S.H. Ryu, T.G. Lee, et al., Proteomic analysis of tumor necrosis factor--induced secretome of human adipose tissue-derived mesenchymal stem cells , J. Proteome Res. 9 (2010) 1754–1762.
- S. Bruno, C. Grange, M.C. Deregibus, R.A. Calogero, S. Saviozzi, F. Collino, et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury, J. Am. Soc. Nephrol. 20 (2009) 1053–1067.
- M. Yá˜nez-Mó, P.R.-M. Siljander, Z. Andreu, A.B. Zavec, F.E. Borràs, E.I. Buzas, et al. Biological properties of extracellular vesicles and their physiological functions (2015).
- C. Lawson, J.M. Vicencio, D.M. Yellon, S.M. Davidson, Microvesicles and exosomes: new players in metabolic and cardiovascular disease , J. Endocrinol. 228 (2016) R57–R71.
- A.G. Thompson, E. Gray, S.M. Heman-Ackah, I. Mager, K. Talbot, S.E. Andaloussi, et al., Extracellular vesicles in neurodegenerative diseas—pathogenesis to biomarkers, Nat. Rev. Neurol. 12 (2016) 346–357.
- I.E.M. Bank, L. Timmers, C.M. Gijsberts, Y.-N. Zhang, A. Mosterd, J.-W. Wang, et al., The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease , Expert Rev. Mol. Diagn. 15 (2015) 1577–1588.
- T. Kato, S. Miyaki, H. Ishitobi, Y. Nakamura, T. Nakasa, M.K. Lotz, et al., Exosomes from IL-1 stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes , Arthritis. Res. Ther. 16 (2014) 1–11.
- R.W.Y. Yeo, S.K. Lim, Exosomes and their therapeutic applications, in: C. Gunther, A. Hauser, R. Huss (Eds.), Advances in Pharmaceutical Cell TherapyPrinciples of Cell-Based Biopharmaceuticals, World Scientific, Singapore, 2015, pp. 477–491.
- X. Qi, J. Zhang, H. Yuan, Z. Xu, Q. Li, X. Niu, et al., Exosomes secreted by human-Induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats , Int. J. Biol. Sci. 12 (2016) 836–849.
- R.C. Lai, F. Arslan, S.S. Tan, B. Tan, A. Choo, M.M. Lee, et al., Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles , J. Mol. Cell. Cardiol. 48 (2010) 1215–1224.
- Y. Zhou, H. Xu, W. Xu, B. Wang, H. Wu, Y. Tao, et al., Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro , Stem Cell Res. Ther. 4 (2013) 1–13.
- Y. Qin, L. Wang, Z. Gao, G. Chen, C. Zhang, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo , Sci. Rep. 6 (2016) 21961.
- M. Nakano, K. Nagaishi, N. Konari, Y. Saito, T. Chikenji, Y. Mizue, et al., Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes , Sci. Rep. 6 (2016) 24805.
- K. Nagaishi, Y. Mizue, T. Chikenji, M. Otani, M. Nakano, N. Konari, et al., Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes , Sci. Rep. 6 (2016) 34842.
- S.R. Baglio, K. Rooijers, D. Koppers-Lalic, F.J. Verweij, M. Pérez Lanzón, N. Zini, et al., Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species , Stem Cell Res. Ther. 6 (2015) 1–20.
- T. Chen, R. Yeo, F. Arslan, Y. Yin, S. Tan, Efficiency of exosome production correlates inversely with the developmental maturity of MSC donor, J. Stem Cell Res. Ther. 3 (2013) 2.
- R.C. Lai, S.S. Tan, B.J. Teh, S.K. Sze, F. Arslan, D.P. de Kleijn, et al., Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome , Int. J. Proteomics 2012 (2012) 971907.
- T.S. Chen, R.C. Lai, M.M. Lee, A.B.H. Choo, C.N. Lee, S.K. Lim, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs , Nucleic Acids Res. 38 (2010) 215–224.
- R.W. Yeo, R.C. Lai, K.H. Tan, S.K. Lim, Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell, J. Circ. Biomark. 1 (2013) 7.
- R.C. Lai, R.W. Yeo, S.K. Lim, Mesenchymal stem cell exosomes, Semin. Cell Dev. Biol. 40 (2015) 82–88.
- B. Zhang, R.W. Yeo, K.H. Tan, S.K. Lim, Focus on extracellular vesicles: therapeutic potential of stem cell-derived extracellular vesicles , Int. J. Mol. Sci. 17 (2016) 174.
- Hu G-w, Q. Li, X. Niu, B. Hu, J. Liu, Zhou S-m, et al., Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice , Stem Cell Res. Ther. 6 (2015) 1–15.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li, et al., Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis , J. Transl. Med. 13 (2015) 1–14.
- B. Zhang, M. Wang, A. Gong, X. Zhang, X. Wu, Y. Zhu, et al., HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing, Stem Cells 33 (2015) 2158–2168.
- B. Zhang, Y. Yin, R.C. Lai, S.S. Tan, A.B.H. Choo, S.K. Lim, Mesenchymal stem cells secrete immunologically active exosomes , Stem Cells Dev. 23 (2013) 1233–1244.
- C.Y. Tan, R.C. Lai, W. Wong, Y.Y. Dan, S.-K. Lim, H.K. Ho, Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models , Stem Cell Res. Ther. 5 (2014) 1–14.
- C. Lee, S.A. Mitsialis, M. Aslam, S.H. Vitali, E. Vergadi, G. Konstantinou, et al., Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension , Circulation 126 (2012) 2601–2611.
- B. Yu, H. Shao, C. Su, Y. Jiang, X. Chen, L. Bai, et al., Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1 , Sci. Rep. 6 (2016) 34562.
- Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof of concept clinical trial. Stem Cells. 2014;32(5):1254–66.
- Vega, Aurelio, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
- Davatchi F, Sadeghi-Abdollahi B, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–5
- Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case- controlled study. Int Orthop. 2014;38(9):1811–1818
- Galli D, Vitale M, Vaccarezza M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:6.
- Beitzel K, Solovyova O, Cote MP, et al. The future role of mesenchymal Stem cells in The management of shoulder disorders . Arthroscopy. 2013;29(10):1702–1711.
- Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190.
- Malda, Jos, et al. " Extracellular vesicles [mdash] new tool for joint repair and regeneration. " Nature Reviews Rheumatology (2016).
Further References about PRP
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4(1):52–62.
Extras
- Xu, Ming, et al. " Transplanted senescent cells induce an osteoarthritis-like condition in mice. " The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (2016): glw154.
- McCulloch, Kendal, Gary J. Litherland, and Taranjit Singh Rai. " Cellular senescence in osteoarthritis pathology ." Aging Cell (2017).
Patient Services at ANOVA Institute for Regenerative Medicine
- Located in the center of Germany, quick access by car or train from anywhere in Europe
- Simple access worldwide, less than 20 minutes from Frankfurt Airport
- Individualized therapy with state-of-the-art stem cell products
- Individually planned diagnostic work-up which include world-class MRI and CT scans
- German high quality standard on safety and quality assurance
- Personal service with friendly, dedicated Patient Care Managers
- Scientific collaborations with academic institutions to assure you the latest regenerative medical programs