Promising New Treatment for Diabetes (Type 1 and Type 2) with Mesenchymal Stem Cells (Secretome) at ANOVA IRM in Offenbach, Germany
Diabetes mellitus is a chronic disease in which the body cannot properly regulate blood sugar. Type 1 Diabetes is an autoimmune condition where the immune system destroys the insulin-producing beta cells in the pancreas, leading to little or no insulin production. Type 2 Diabetes is a metabolic disorder in which the body’s cells become resistant to insulin and the pancreas cannot keep up by producing enough insulin. In both types, blood glucose levels become too high, causing symptoms like excessive thirst, frequent urination, fatigue, and unintended weight loss. Over time, high blood sugar can damage the body’s organs, leading to serious complications such as heart disease, nerve damage, kidney failure, and vision loss. Diabetes affects hundreds of millions of people worldwide and currently has no simple cure – management with medications (like insulin) and lifestyle changes is required for life.
ANOVA Institute for Regenerative Medicine (ANOVA IRM) in Germany offers an innovative experimental therapy for Type 1 and Type 2 Diabetes using Mesenchymal Stem Cell (MSCs) secretome. This therapy aims to modulate the immune system and regenerate pancreatic function – addressing the root causes of diabetes rather than just controlling blood sugar. ANOVA’s Stem Cell Secretome is a proprietary cell-free product derived from a patient’s own MSCs, containing the beneficial factors (exosomes, growth factors, cytokines) that MSCs release. In Europe, ANOVA IRM is a pioneer and the first clinic authorized to provide this autologous MSC secretome treatment. According to scientific research and early clinical trials, MSC-based therapies have shown potential to calm autoimmune attacks and support the repair of insulin-producing cells. All forms of diabetes may potentially be treated – for Type 1, by reducing the immune system’s attack on the pancreas and preserving/restoring insulin production; for Type 2, by reducing systemic inflammation, improving insulin sensitivity, and aiding tissue repair in organs affected by diabetes.
To learn more about our personalized stem cell-based diabetes treatment plans and to discuss if you are a candidate, please contact ANOVA IRM – we are happy to schedule an initial consultation and answer any questions. Below we provide a comprehensive overview of diabetes, current treatments, our MSC therapy approach, and what patients can expect when seeking treatment at ANOVA IRM in Offenbach (near Frankfurt am Main Airport), Germany.
Diabetes Mellitus Overview – Causes, Symptoms, Complications, and Treatments
On this page, we inform you about diabetes and introduce the stem cell-based therapy offered at ANOVA IRM. You will find an overview of the causes, symptoms, and complications of Type 1 and Type 2 diabetes, the conventional therapy options available, and details of our mesenchymal stem cell (MSC) therapy including how it works, the treatment process, and frequently asked questions.
Jump directly to a topic:
Conventional Diabetes Therapies
Current standard treatments for diabetes focus on managing blood glucose levels and mitigating symptoms. They do not cure the disease or fully stop its progression:
- Type 1 Diabetes: Since the body produces little to no insulin, patients must take insulin to survive – either through multiple daily injections or an insulin pump. Blood sugar must be monitored frequently. Careful diet and lifestyle management are also crucial. In some cases, physicians may use insulin pump technology or continuous glucose monitors to help maintain control. There are experimental efforts to immunologically modulate Type 1 (for example, medications to suppress the autoimmune attack), but no widely available therapy yet prevents the immune system from attacking the pancreas. Aside from a rare pancreas or islet cell transplant, which involves major surgery and immunosuppressive drugs, there is no way to restore the body’s own insulin production. Thus, Type 1 diabetes is managed life-long by replacing insulin and trying to maintain blood sugar in a safe range. Even with excellent management, patients may experience episodes of high or low blood sugar and remain at risk for long-term complications.
- Type 2 Diabetes: Treatment typically begins with lifestyle modifications – healthy diet, weight loss, and regular exercise – which can significantly improve blood sugar control. Many patients also take oral medications such as metformin (to reduce glucose production and improve insulin sensitivity) or other drug classes (sulfonylureas, SGLT2 inhibitors, GLP-1 agonists, etc.) to help the body use insulin better or lower blood sugar by other mechanisms. As Type 2 diabetes progresses, the pancreas may produce less insulin over time; some patients eventually require insulin injections as well. These conventional therapies can effectively lower blood sugar and reduce symptoms. However, they generally do not reverse the underlying insulin resistance or loss of beta-cell function – they manage the disease rather than cure it. Many people with Type 2 diabetes face escalating treatment regimens over the years if the disease progresses.
- Managing Complications: Alongside blood sugar control, conventional therapy involves treating or preventing complications. For example, patients may take medications for blood pressure or cholesterol to reduce cardiovascular risks, undergo regular eye exams for retinopathy, use pain management for neuropathy, etc. Education on foot care and routine medical check-ups are also standard, since complications like foot ulcers can be caught early with proper care.
While these conventional therapies are essential and have drastically improved outcomes for people with diabetes, they have limitations. Insulin and medications must be taken indefinitely and require vigilant daily management. High blood sugar can still occur and cause ongoing damage. Importantly, none of the standard treatments can repair the lost insulin-producing cells or fundamentally alter the course of the disease. This is why researchers and clinics like ANOVA IRM are exploring regenerative approaches such as stem cell therapy – to address the disease at a deeper level.
Stem Cell Therapy for Diabetes – How Can MSCs Help?
Given the challenges of conventional treatments, stem cell-based therapies have emerged as a promising investigational approach for diabetes. Mesenchymal stem cells (MSCs) have special properties that may address the root problems in both Type 1 and Type 2 diabetes:
- Immunomodulation: MSCs can interact with immune cells and release natural signals that reduce harmful immune responses. In Type 1 diabetes, this could mean dampening the misguided autoimmune attack on pancreatic beta cells, potentially preserving any remaining insulin-producing cells from further destruction. In Type 2 diabetes, MSCs’ anti-inflammatory effects might improve chronic inflammation associated with insulin resistance. Essentially, MSCs act as immune regulators, helping to inhibit immune “over-reactions” while promoting a healthier immune balance.
- Regeneration of Pancreatic Function: MSCs secrete a variety of growth factors and cytokines (collectively known as the secretome) that stimulate tissue repair. Research indicates that these secreted factors can facilitate regeneration of beta cells in the pancreas and support the formation of new blood vessels (improving pancreatic blood supply). In laboratory and animal studies, MSC therapy has been shown to ameliorate hyperglycemia and encourage pancreatic islet repair by creating a more regenerative environment. This suggests that MSCs might help the pancreas recover some capacity to produce insulin. While it’s not expected to fully reverse long-standing diabetes, even partial restoration of insulin production can significantly improve disease control.
- Improving Metabolic Control and Complications: By modulating the immune system and promoting repair, MSC therapy can have systemic benefits. Clinical studies have observed that patients receiving MSC treatments often have better blood sugar control – for example, lower HbA1c levels (a measure of 3-month blood glucose average) – and require less insulin to manage their diabetes after therapy. Some trials reported that a portion of patients were able to reduce their daily insulin dose by more than 50%, and a few even became insulin-independent for some time. Additionally, MSCs’ trophic (healing) factors may aid in repairing other tissues damaged by diabetes. For instance, MSC treatments have neurotrophic and pro-angiogenic effects – they can support nerve repair and improve blood circulation. This raises the possibility of improvements in diabetic complications such as neuropathy (nerve damage) and difficult wound healing. Indeed, preclinical studies have shown MSC-derived exosomes helped heal diabetic nerve damage and enhanced muscle and fat metabolism in models of diabetes.
It’s important to stress that MSC therapy for diabetes is still experimental, but results so far have been encouraging. In clinical research (including multiple trials in humans), MSC treatments have demonstrated the following outcomes:
- Better blood glucose control: Patients treated with MSCs often show a significant drop in HbA1c (glycated hemoglobin) from baseline, indicating improved long-term glucose levels.
- Reduced insulin requirements: Several studies report that after MSC therapy, patients’ need for injected insulin decreases. Some patients reduced their insulin dose significantly, and some Type 1 patients even became insulin-free for a period.
- Improved beta-cell function: Biomarkers of the pancreas’s insulin production, such as C-peptide levels, have been observed to increase in some patients after MSC therapy, suggesting restoration of beta cell function.
- Safety and tolerability: No major side effects have been reported in published trials. MSC infusions were generally well-tolerated, with only minor transient reactions noted in a few cases. Autologous therapy eliminates rejection risk.
Most importantly, MSC therapy is not a cure for diabetes (at least not with current knowledge), and it is not guaranteed to work for every patient. However, these early findings suggest it can be a disease-modifying therapy – potentially slowing down the disease or partially reversing some of its damage. By reducing autoimmunity and stimulating regeneration, MSC treatments could give patients better control of their diabetes and improve their quality of life.
Stem cell research over the past decades has advanced to the point that ANOVA IRM, a German stem cell clinic in the heart of Europe, is able to translate these findings into a treatment program. We offer a novel therapeutic approach: ANOVA’s Stem Cell Secretome – a cell-free MSC therapy – as a promising option for patients with diabetes. This treatment leverages the natural healing and immunomodulatory powers of MSCs without actually transplanting live cells. ANOVA IRM has received the necessary regulatory approvals in Germany to provide this therapy in a controlled clinical setting, ensuring the highest quality and safety standards.
If you are interested in our stem cell-based diabetes therapy, check your eligibility and apply for treatment or simply reach out to us for more information. Our medical team will review your case individually and guide you through your options. Please use our contact form or call us for further information and to schedule an appointment.
Comparison: Conventional Therapies vs. MSC-Based Therapy
The table below highlights key differences between traditional diabetes treatments and the experimental MSC-based stem cell therapy offered at ANOVA IRM:
Stem Cell Treatment for Diabetes at ANOVA IRM – Secretome/Exosomes of MSC
Potency Hypothesis of Stem Cell Therapies
Why might stem cells help where other treatments do not? The potency hypothesis of MSC therapy suggests that MSCs have a unique ability to address complex conditions like diabetes through multiple mechanisms. Stem cells can “sense” signals of injury or inflammation in the body and respond by secreting factors that orchestrate healing. They communicate with immune cells that drive inflammation and, through still not fully understood natural mechanisms, can inhibit harmful immune over-reactions. At the same time, MSCs release growth factors that stimulate the regeneration of tissues, counteracting the loss of function caused by disease. In diabetes, this dual action means MSCs could simultaneously calm the immune attack (in Type 1) and promote repair of pancreatic islets or other affected tissues. Unlike single-target drugs, stem cells work in a holistic, dynamic way – they modulate the body’s environment to foster healing. It’s this broad spectrum of action that makes MSCs a promising tool for a multifactorial disease like diabetes.
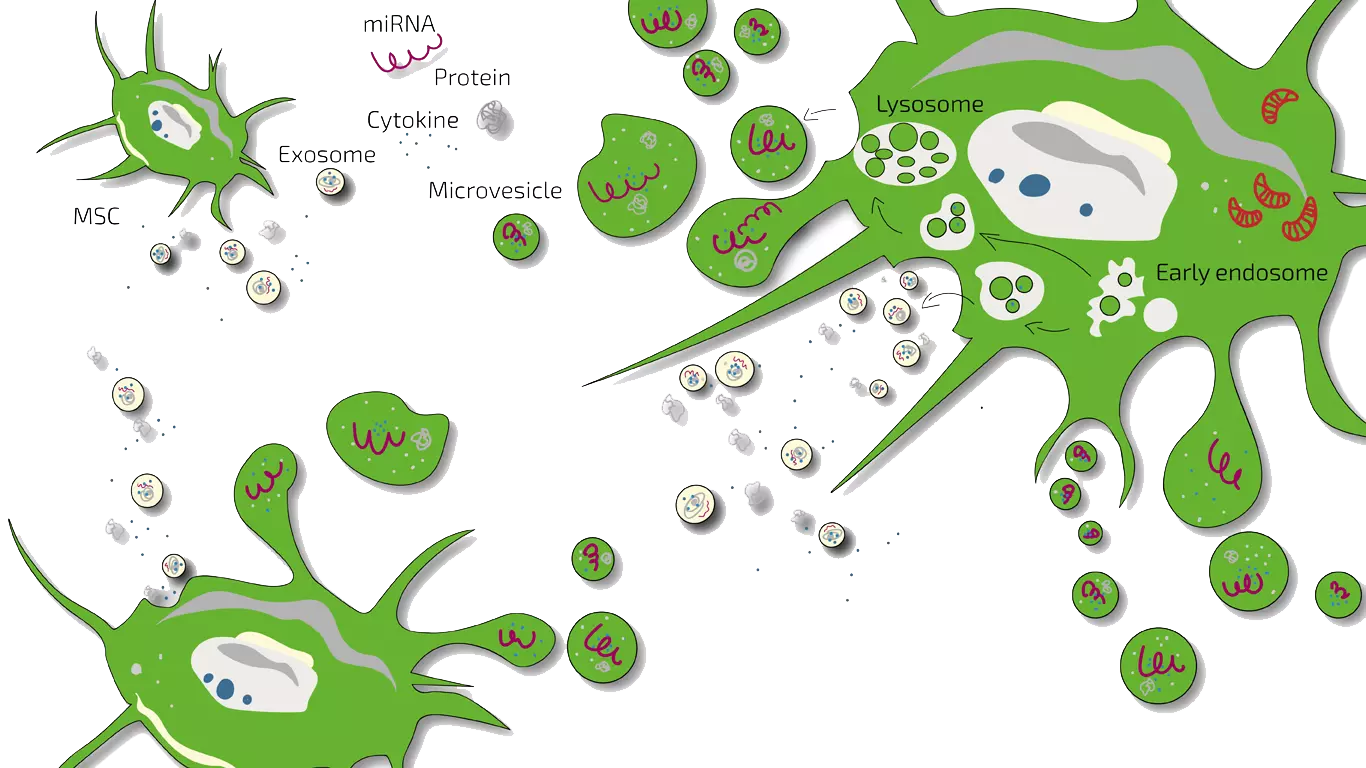
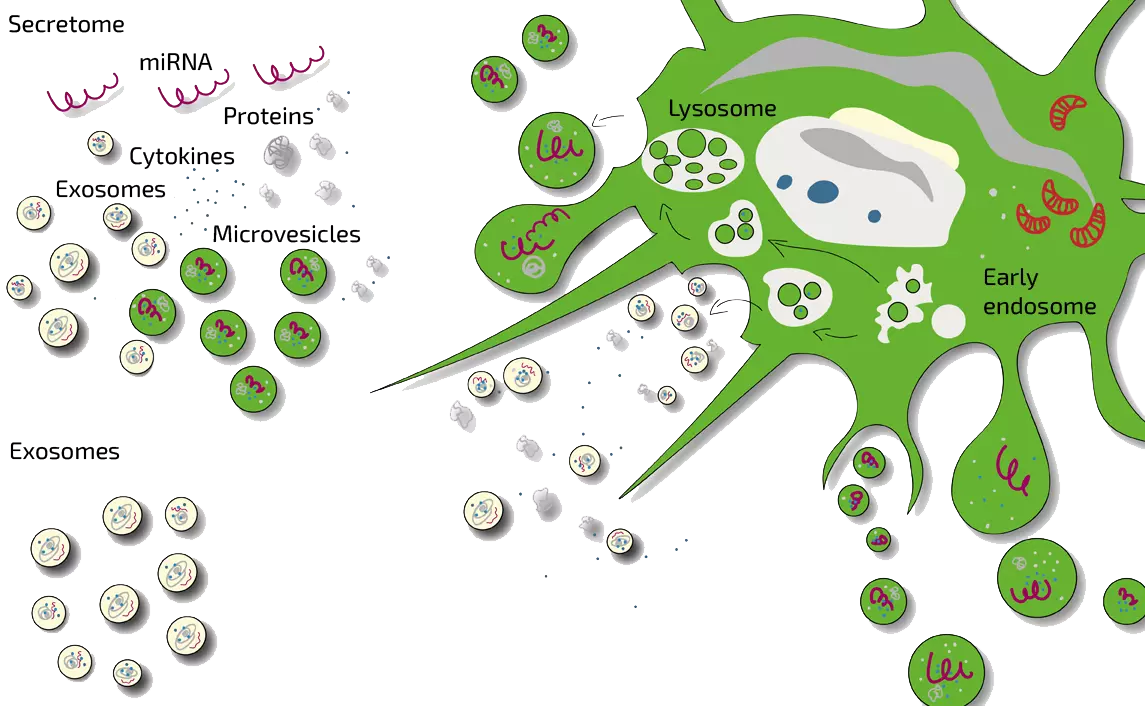
MSEC – Mesenchymal Stem Cell Secretome (Exosomes) – Autologous Therapy
ANOVA IRM’s approach for treating diabetes is to harness MSCs’ benefits without injecting live cells. Instead, we use MSEC (Mesenchymal Stem Cell Exosome/Secretome) therapy – a cell-free product derived from your own MSCs. Here’s how it works in practice:
- We obtain a source of MSCs from the patient’s adipose tissue (body fat) via a mini-liposuction, a brief and minimally invasive procedure.
- In our advanced GMP-certified laboratory, we isolate and culture these cells to expand their numbers and stimulate them to produce the secretome (exosomes, growth factors, cytokines).
- After production, the cellular material is removed, leaving a purified MSC secretome product, which is quality-tested.
- The MSC secretome is delivered back to the patient via injections or infusions (typically intravenous for diabetes).
Worldwide, ANOVA IRM was the first stem cell clinic to obtain legal permission from regulatory authorities to produce and use this kind of autologous exosome-rich secretome therapy. Our MSC secretome is provided under strict oversight for quality and safety, meeting pharmaceutical-grade manufacturing standards.
The main advantage of secretome therapy (MSEC) over traditional cell therapy is convenience and consistency: the secretome product can be frozen and stored without losing its effectiveness. One mini-liposuction can yield 10–20 individual treatment doses, allowing for treatment cycles over many months without repeated liposuction procedures.
What exactly are exosomes and secretome? Exosomes are nano-sized vesicles carrying signals. The secretome includes exosomes and all other soluble factors a cell secretes. ANOVA’s product is a whole secretome, preserving the full spectrum of regenerative signals.
Please note that this treatment is not a cure for diabetes; it is an experimental, potential disease-modifying therapy. Patients must continue standard diabetes care. The therapy requires regular travel to our clinic in Offenbach, Germany, for multiple sessions.
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
Therapy Workflow for Diabetes – Treatment Process at ANOVA IRM
The treatment process for undergoing MSC secretome therapy at ANOVA IRM is carefully structured and typically occurs in several phases. The overall workflow is similar for both Type 1 and Type 2 diabetes patients. Here is what you can generally expect:
- Initial Evaluation and Consultation: Remote review of medical records, consultation (phone/video) to assess suitability and answer questions.
- Pre-Treatment Work-up: Personalized treatment plan, necessary diagnostic tests, cost estimate, regulatory paperwork.
- Tissue Harvest (Mini-Liposuction Procedure): Visit Offenbach, Germany (typically 2 days). Minor procedure under local anesthesia/sedation to extract fat tissue.
- MSC Processing and Secretome Production: Lab isolates MSCs, cultures them, produces and quality-tests the secretome (~4 weeks, patient returns home).
- Treatment Delivery (Secretome Infusions/Injections): Return visits to ANOVA IRM for treatment sessions (e.g., IV infusions). Multiple sessions scheduled over months (e.g., every 4-8 weeks).
- Follow-Up and Monitoring: Track progress (HbA1c, insulin needs, C-peptide, symptoms), coordinate with local doctors, monitor for side effects, evaluate need for further treatment.
In summary, the process involves an initial remote evaluation, a short visit for cell harvesting, a wait of a few weeks, then multiple return visits for infusions. The diagram below summarizes the treatment workflow:
Every patient’s case is unique, so the above timeline can vary. We strive to tailor the process to your needs and minimize inconvenience. Our team assists with logistics like scheduling, travel advice, and visa documentation if needed.
How Much Does Stem Cell Treatment Cost?
We provide individualized treatments, so the cost varies based on the number of doses, complexity, etc. A personalized cost estimate is provided after evaluation. As a guideline, a full MSC secretome therapy program for diabetes costs in the five-figure range (well above ten thousand Euros). This covers cell harvesting, lab processing, quality controls, administration of multiple doses, and medical supervision. We are transparent about costs upfront.
Does My Health Insurance Cover the Therapy?
Unfortunately, health insurance companies do not currently cover experimental treatments like MSC secretome therapy. It is not yet standard care. Patients must typically bear the costs out-of-pocket, including travel. We provide detailed invoices for potential submission to tax authorities or healthcare savings accounts where applicable. ANOVA IRM is a private clinic requiring direct payment. We can provide information if you seek crowdfunding or other funding options.
(For any cost or payment-related inquiries, feel free to contact our patient care coordinators via the contact form.)
Treating Diabetes at ANOVA IRM – Our Approach
At ANOVA IRM, our mission is to provide personalized, high-quality regenerative treatments responsibly. Our approach includes:
- Combination of Established and Novel Therapies: We advise continuing conventional therapy alongside regenerative treatment, working with your primary doctors.
- Thorough Diagnostics and Monitoring: Comprehensive work-ups establish baselines and track progress objectively, allowing for personalized adjustments.
- Evidence-Based Protocols: Treatment guidelines are based on scientific evidence, published studies, and internal research. Realistic expectations are set.
- Ethics and Patient Safety: We adhere to strict ethical standards, conduct risk-benefit analyses, obtain informed consent, and prioritize well-being. Our clinic operates under stringent German healthcare regulations.
Make an appointment today to learn more about your treatment options at ANOVA IRM. Our team is dedicated to offering cutting-edge therapy with compassion and professionalism.
(Use our contact form or phone number to get in touch – we typically respond within 1–2 business days.)
Stem Cell-Based Treatment for Diabetes: Your Personalized, High-Quality Therapy
ANOVA IRM leverages the latest scientific knowledge for effective stem cell therapies:
- Quality and Safety: Legally authorized, bi-annually audited processes, pharmaceutical-grade quality control exceeding minimum requirements.
- State-of-the-Art Laboratory: Cutting-edge facility, standardized procedures optimized for potency, highly trained staff, advanced characterization methods.
- Advanced Diagnostics Integration: Modern diagnostics (blood tests, imaging if needed) used throughout to monitor progress and guide therapy.
- Personalized Treatment and Follow-up: Therapy plan tailored to individual needs and response. Continuous assessment and long-term follow-up.
We strive to offer the best possible care in stem cell therapy for diabetes in a world-class medical setting. We understand the significance of seeking experimental treatment abroad and support you throughout the process.
Important: While optimistic, we remain realistic. This treatment is innovative, and results vary. We cannot guarantee improvement for every patient but promise to apply our expertise fully. If stem cell therapy is unsuitable, we will provide honest guidance.
To find out more about your specific case, please contact us for a detailed consultation. ANOVA IRM is a private clinic specializing in regenerative medicine since 2016.
FAQ: Stem Cell-Based Therapies for Diabetes
Below we address some frequently asked questions patients have about diabetes and the MSC therapy offered at ANOVA IRM.
What is Type 1 Diabetes?
Type 1 Diabetes (T1D) is an autoimmune disease where the immune system destroys insulin-producing beta cells in the pancreas, leading to little or no insulin production. Glucose builds up in the blood (hyperglycemia). Symptoms often appear suddenly and include extreme thirst, frequent urination, weight loss, fatigue, and blurred vision. Untreated, it can lead to diabetic ketoacidosis (DKA). T1D often starts in childhood/young adulthood but can occur at any age. It requires lifelong insulin therapy and monitoring. There is no cure or prevention method currently.
What is Type 2 Diabetes?
Type 2 Diabetes (T2D) is the most common form (~90% of cases), characterized by insulin resistance (cells don't respond well to insulin) and eventually reduced insulin production. Risk factors include overweight/obesity, inactivity, family history, and ethnicity. It usually develops gradually in adults but is increasing in younger people. Symptoms are similar to T1D but milder initially; some have no symptoms for years. T2D is progressive and often requires lifestyle changes, oral medications, and sometimes insulin. It can often be prevented or delayed with healthy habits.
What are the Symptoms of Diabetes?
Common signs for both types include:
- Excessive thirst (polydipsia) and dry mouth.
- Frequent urination (polyuria).
- Extreme hunger (polyphagia).
- Fatigue and weakness.
- Blurred vision.
- Slow-healing wounds or frequent infections.
- Unexplained weight loss (more common in T1D).
- Numbness or tingling in hands or feet (neuropathy).
T1D symptoms usually appear quickly; T2D symptoms develop slowly. Seek medical evaluation if you experience these signs.
What are the Complications of Diabetes?
Chronic high blood sugar damages blood vessels and nerves, leading to:
- Acute Complications: Diabetic Ketoacidosis (DKA, mainly T1D), Hyperosmolar Hyperglycemic State (HHS, mainly T2D), severe hypoglycemia (low blood sugar from treatment). These are emergencies.
- Long-Term Complications: Cardiovascular disease (heart attack, stroke), neuropathy (nerve damage, especially feet), nephropathy (kidney damage/failure), retinopathy (eye damage/blindness), foot problems (ulcers, infections, amputation risk), skin conditions, digestive issues (gastroparesis), sexual dysfunction, hearing loss, dental problems.
Good blood sugar control significantly reduces complication risk. MSC therapy aims to improve control and potentially reduce long-term damage.
How does the MSC Secretome Therapy actually work for Diabetes?
The MSC secretome contains factors that circulate and act as messengers. In T1D, they may calm the immune attack on the pancreas and stimulate beta cell regeneration/function. In T2D, anti-inflammatory effects can improve insulin sensitivity, and factors may promote blood vessel formation, enhancing glucose uptake and pancreatic function. The secretome acts systemically, potentially helping the pancreas, immune system, peripheral tissues, blood vessels, and nerves. Effects develop over weeks to months, requiring a series of treatments.
Is MSC Stem Cell Therapy a Cure for Diabetes?
No, MSC therapy is not a guaranteed cure. It's an experimental therapy aiming to improve the condition, potentially induce partial remission, but not eliminate diabetes permanently. Some T1D patients in trials became temporarily insulin-independent but usually resumed insulin later (often at lower doses). Effects might wane, possibly requiring booster treatments. It should be viewed as an additional tool to manage diabetes, not a replacement for standard care or a miracle cure. Individual response varies.
Who is Eligible for Stem Cell Therapy for Diabetes at ANOVA IRM?
Eligibility is case-by-case, generally:
- Adults (18+) with confirmed T1D or T2D.
- Medically stable for travel and minor procedure.
- Both recent-onset and long-term T1D considered.
- T2D patients with difficulty controlling diabetes or complications may be candidates.
- No contraindications (active cancer, pregnancy, severe unstable illness, active infection, etc.).
- Able to consent and participate, with realistic expectations.
Contact us with medical information for an assessment.
What does the treatment involve – do I have to get surgery?
It involves a mini-liposuction (minor procedure under local anesthesia/sedation to get fat), blood draws, lab processing (patient not present), and then intravenous infusions of the secretome (outpatient sessions). No major surgery is involved. The liposuction has quick recovery. Infusions are like getting an IV medicine drip.
How often do I need to come to Germany for this treatment?
You'll make an initial 2-day trip for the liposuction. After ~4 weeks, you'll return for infusion sessions. A typical schedule might involve 4-6 visits in the first year (e.g., every 4-8 weeks). Each visit might be 1-2 days. We can adjust scheduling for international travelers (e.g., clustering infusions). The stored secretome lasts up to 2 years, allowing flexibility.
How soon might I see results, and how will I know if it’s working?
Subjective improvements (energy, fewer glucose swings) might occur within weeks. Objective measures (lower HbA1c, reduced insulin needs, increased C-peptide) typically take 3-6 months. Signs it's working include better daily readings, less glucose variability, lower insulin dose, improved lab tests (HbA1c, C-peptide), and relief from symptoms like neuropathy or fatigue. We monitor progress closely, with major evaluations often at 6 months.
Is the MSC therapy safe? What are the risks and side effects?
MSC therapy has a strong safety profile. Using autologous material minimizes rejection/allergic reactions. No serious adverse events directly linked to MSC/secretome therapy reported in major trials.
- Infusion: Minor, transient side effects like mild fever, chills, headache, or nausea are possible but uncommon.
- Liposuction: Small risks like bruising, soreness, numbness, or very rarely infection or bleeding.
- Hypoglycemia: Low blood sugar can occur if insulin dose isn't reduced as body function improves (manageable with monitoring).
- Long-term: No known long-term risks; secretome is cell-free, avoiding risks of unwanted cell growth.
The main "risks" are often the financial cost, travel burden, and uncertainty of benefit, rather than physical harm.
How does ANOVA IRM’s approach differ from other treatments or clinics?
ANOVA IRM distinguishes itself through:
- Innovative Secretome Therapy: Pioneering cell-free approach enhancing safety and potency.
- Autologous Therapy & High Standards: Using patient's own cells under GMP-like conditions and strict German regulatory oversight.
- Holistic Care & Follow-up: Integrated treatment, diagnostics, collaboration with other doctors, long-term monitoring.
- Experienced Team: World-renowned experts in regenerative medicine.
- Legal & Ethical Compliance: Operating transparently within legal frameworks.
- Combination Therapies: Offering multiple regenerative tools (BMC, PRP, Secretome) for tailored solutions.
How can I get started or learn more about receiving treatment?
Contact us via our online form, email, or phone. We'll request medical records (doctor's summary, labs like HbA1c/C-peptide, medication list, complication reports). Our team reviews your case, followed by a remote consultation with a doctor to discuss suitability, the process, and answer questions. If eligible and you decide to proceed, we provide a treatment proposal, consent forms, and assist with scheduling and travel logistics. A dedicated patient liaison supports you throughout.
We hope this information page has been helpful. Diabetes is challenging, but new therapies like MSC secretome treatment offer hope. At ANOVA IRM, we combine science with compassionate care. Please contact us with further questions.
Ready to take the next step? – Reach out to us today to schedule your personalized consultation.
Sources and Literature
(A selection of scientific references and sources that inform our therapy and the content above):
- Habiba UE et al., 2024 – Frontiers in Endocrinology: Meta-analysis showing MSC therapy improved HbA1c and reduced insulin needs in T1D and T2D without serious adverse effects. (Links: PubMed)
- Yang L et al., 2021 – Stem Cell Research & Therapy: Meta-analysis showing improved glycemic outcomes after MSC transplant, especially in T1D, with some T2D achieving insulin independence. (Links: PMC)
- Khatri R et al., 2020 – Stem Cell Research & Therapy: Preclinical study showing intrapancreatic MSCs stimulated islet regeneration. (Links: PMC)
- Zhang H, et al., 2015 - PubMed: Study suggesting MSCs promote pancreatic β-cell regeneration via FoxO1 pathway downregulation. (Link: PubMed)
- American Diabetes Association (ADA): General diabetes information. (Links: Understanding Type 1 Diabetes | ADA)
- Centers for Disease Control and Prevention (CDC): General diabetes information. (Links: Type 2 Diabetes | Diabetes | CDC)
- Cleveland Clinic: General diabetes information. (Link: Diabetes: What It Is, Causes, Symptoms, Treatment & Types)
- ANOVA IRM Website Pages: Information on Secretome/Exosomes, Multiple Sclerosis treatment (used for comparative info on process/cost/insurance). (Links: Stem Cell Secretome (SCS) | ANOVA IRM Germany, Multiple Sclerosis (MS) | ANOVA IRM Germany)
(For a full list of references or further reading, please contact ANOVA IRM or see our “Literature” section on the website.)
Further References for MSC, BMC, Stemcell Secretome and EVs
- Georg Hansmann, Philippe Chouvarine, Franziska Diekmann, Martin Giera, Markus Ralser, Michael Mülleder, Constantin von Kaisenberg, Harald Bertram, Ekaterina Legchenko & Ralf Hass "Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension". Nature Cardiovascular Research volume 1, pages568–576 (2022).
- Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis . Arthritis Rheum. 2003;48:3464–74.
- Lee KB, Hui JH, Song IC, Ardany L, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cell. 2007;25:2964–71.
- Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy . 2009;25(12):1391–400.
- Black L, Gaynor J, Adams C, et al. Effect of intra-articular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200.
- Centeno C, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–53.
- Centeno C, Kisiday J, Freeman M, et al. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: a case study. Pain Physician. 2006;9:253–6.
- Centeno C, Schultz J, Cheever M. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell. 2011;5(1):81–93.
- Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose derived stem cells: a case series. J Med Case Rep. 2001;5:296.
- Kuroda R, Ishida K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31.
- Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422–8.
- Saw KY et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94.
- Vangsness CT, Farr J, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg. 2014;96(2):90–8.
- Freitag, Julien, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy–a review. BMC musculoskeletal disorders 17.1 (2016): 230.
- Maumus, Marie, Christian Jorgensen, and Danièle Noël. " Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. " Biochimie 95.12 (2013): 2229-2234.
- Dostert, Gabriel, et al. " How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication?. " Frontiers in Cell and Developmental Biology 5 (2017).
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- Toh, Wei Seong, et al. " MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. " Seminars in Cell & Developmental Biology. Academic Press, 2016.
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- S. Koelling, J. Kruegel, M. Irmer, J.R. Path, B. Sadowski, X. Miro, et al., Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis , Cell Stem Cell 4 (2009) 324–335.
- B.A. Jones, M. Pei, Synovium-Derived stem cells: a tissue-Specific stem cell for cartilage engineering and regeneration , Tissue Eng. B: Rev. 18 (2012) 301–311.
- W. Ando, J.J. Kutcher, R. Krawetz, A. Sen, N. Nakamura, C.B. Frank, et al., Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage, Cytotherapy 16 (2014) 776–788.
- K.B.L. Lee, J.H.P. Hui, I.C. Song, L. Ardany, E.H. Lee, Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model, Stem Cells 25 (2007) 2964–2971.
- W.-L. Fu, C.-Y. Zhou, J.-K. Yu, A new source of mesenchymal stem cells for articular cartilage repair: mSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model , Am. J. Sports Med. 42 (2014) 592–601.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, et al., Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration , Biomaterials 33 (2012) 7008–7018.
- E.-R. Chiang, H.-L. Ma, J.-P. Wang, C.-L. Liu, T.-H. Chen, S.-C. Hung, Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits , PLoS One 11 (2016) e0149835.
- H. Nejadnik, J.H. Hui, E.P. Feng Choong, B.-C. Tai, E.H. Lee, Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study , Am. J. Sports Med. 38 (2010) 1110–1116.
- I. Sekiya, T. Muneta, M. Horie, H. Koga, Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects , Clin. Orthop. Rel. Res. 473 (2015) 2316–2326.
- Y.S. Kim, Y.J. Choi, Y.G. Koh, Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes , Am. J. Sports Med. 43 (2015) 2293–2301.
- W.-L. Fu, Y.-F. Ao, X.-Y. Ke, Z.-Z. Zheng, X. Gong, D. Jiang, et al., Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment , Knee 21 (2014) 609–612.
- Y.-G. Koh, O.-R. Kwon, Y.-S. Kim, Y.-J. Choi, D.-H. Tak, Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial , Arthrosc. J. Arthrosc. Relat. Surg. 32 (2016) 97–109.
- T.S. de Windt, L.A. Vonk, I.C.M. Slaper-Cortenbach, M.P.H. van den Broek, R. Nizak, M.H.P. van Rijen, et al., Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-Stage cartilage repair in humans upon mixture with recycled autologous chondrons , Stem Cells (2016) (n/a-n/a).
- L. da Silva Meirelles, A.M. Fontes, D.T. Covas, A.I. Caplan, Mechanisms involved in the therapeutic properties of mesenchymal stem cells , Cytokine Growth Factor Rev. 20 (2009) 419–427.
- W.S. Toh, C.B. Foldager, M. Pei, J.H.P. Hui, Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration , Stem Cell Rev. Rep. 10 (2014) 686–696.
- R.C. Lai, F. Arslan, M.M. Lee, N.S.K. Sze, A. Choo, T.S. Chen, et al., Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury , Stem Cell Res. 4 (2010) 214–222.
- S. Zhang, W.C. Chu, R.C. Lai, S.K. Lim, J.H.P. Hui, W.S. Toh, Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration, Osteoarthr . Cartil. 24 (2016) 2135–2140.
- S. Zhang, W. Chu, R. Lai, J. Hui, E. Lee, S. Lim, et al., 21 – human mesenchymal stem cell-derived exosomes promote orderly cartilage regeneration in an immunocompetent rat osteochondral defect model , Cytotherapy 18 (2016) S13.
- C.T. Lim, X. Ren, M.H. Afizah, S. Tarigan-Panjaitan, Z. Yang, Y. Wu, et al., Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model
- A. Gobbi, G. Karnatzikos, S.R. Sankineani, One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee , Am. J. Sports Med. 42 (2014) 648–657.
- A. Gobbi, C. Scotti, G. Karnatzikos, A. Mudhigere, M. Castro, G.M. Peretti, One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years , Knee Surg. Sports Traumatol. Arthrosc. (2016) 1–8.
- A. Gobbi, G. Karnatzikos, C. Scotti, V. Mahajan, L. Mazzucco, B. Grigolo, One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-Year follow-up , Cartilage 2 (2011) 286–299.
- K.L. Wong, K.B.L. Lee, B.C. Tai, P. Law, E.H. Lee, J.H.P. Hui, Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up , Arthrosc. J. Arthrosc. Relat. Surg. 29 (2013) 2020–2028.
- J.M. Hare, J.E. Fishman, G. Gerstenblith, et al., Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the poseidon randomized trial, JAMA 308 (2012) 2369–2379.
- L. Wu, J.C.H. Leijten, N. Georgi, J.N. Post, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation , Tissue Eng. A 17 (2011) 1425–1436.
- L. Wu, H.-J. Prins, M.N. Helder, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells in chondrocyte Co-Cultures are independent of culture conditions and cell sources , Tissue Eng. A 18 (2012) 1542–1551.
- S.K. Sze, D.P.V. de Kleijn, R.C. Lai, E. Khia Way Tan, H. Zhao, K.S. Yeo, et al., Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells , Mol. Cell. Proteomics 6 (2007) 1680–1689.
- M.B. Murphy, K. Moncivais, A.I. Caplan, Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine , Exp. Mol. Med. 45 (2013) e54.
- M.J. Lee, J. Kim, M.Y. Kim, Y.-S. Bae, S.H. Ryu, T.G. Lee, et al., Proteomic analysis of tumor necrosis factor--induced secretome of human adipose tissue-derived mesenchymal stem cells , J. Proteome Res. 9 (2010) 1754–1762.
- S. Bruno, C. Grange, M.C. Deregibus, R.A. Calogero, S. Saviozzi, F. Collino, et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury, J. Am. Soc. Nephrol. 20 (2009) 1053–1067.
- M. Yá˜nez-Mó, P.R.-M. Siljander, Z. Andreu, A.B. Zavec, F.E. Borràs, E.I. Buzas, et al. Biological properties of extracellular vesicles and their physiological functions (2015).
- C. Lawson, J.M. Vicencio, D.M. Yellon, S.M. Davidson, Microvesicles and exosomes: new players in metabolic and cardiovascular disease , J. Endocrinol. 228 (2016) R57–R71.
- A.G. Thompson, E. Gray, S.M. Heman-Ackah, I. Mager, K. Talbot, S.E. Andaloussi, et al., Extracellular vesicles in neurodegenerative diseas—pathogenesis to biomarkers, Nat. Rev. Neurol. 12 (2016) 346–357.
- I.E.M. Bank, L. Timmers, C.M. Gijsberts, Y.-N. Zhang, A. Mosterd, J.-W. Wang, et al., The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease , Expert Rev. Mol. Diagn. 15 (2015) 1577–1588.
- T. Kato, S. Miyaki, H. Ishitobi, Y. Nakamura, T. Nakasa, M.K. Lotz, et al., Exosomes from IL-1 stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes , Arthritis. Res. Ther. 16 (2014) 1–11.
- R.W.Y. Yeo, S.K. Lim, Exosomes and their therapeutic applications, in: C. Gunther, A. Hauser, R. Huss (Eds.), Advances in Pharmaceutical Cell TherapyPrinciples of Cell-Based Biopharmaceuticals, World Scientific, Singapore, 2015, pp. 477–491.
- X. Qi, J. Zhang, H. Yuan, Z. Xu, Q. Li, X. Niu, et al., Exosomes secreted by human-Induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats , Int. J. Biol. Sci. 12 (2016) 836–849.
- R.C. Lai, F. Arslan, S.S. Tan, B. Tan, A. Choo, M.M. Lee, et al., Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles , J. Mol. Cell. Cardiol. 48 (2010) 1215–1224.
- Y. Zhou, H. Xu, W. Xu, B. Wang, H. Wu, Y. Tao, et al., Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro , Stem Cell Res. Ther. 4 (2013) 1–13.
- Y. Qin, L. Wang, Z. Gao, G. Chen, C. Zhang, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo , Sci. Rep. 6 (2016) 21961.
- M. Nakano, K. Nagaishi, N. Konari, Y. Saito, T. Chikenji, Y. Mizue, et al., Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes , Sci. Rep. 6 (2016) 24805.
- K. Nagaishi, Y. Mizue, T. Chikenji, M. Otani, M. Nakano, N. Konari, et al., Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes , Sci. Rep. 6 (2016) 34842.
- S.R. Baglio, K. Rooijers, D. Koppers-Lalic, F.J. Verweij, M. Pérez Lanzón, N. Zini, et al., Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species , Stem Cell Res. Ther. 6 (2015) 1–20.
- T. Chen, R. Yeo, F. Arslan, Y. Yin, S. Tan, Efficiency of exosome production correlates inversely with the developmental maturity of MSC donor, J. Stem Cell Res. Ther. 3 (2013) 2.
- R.C. Lai, S.S. Tan, B.J. Teh, S.K. Sze, F. Arslan, D.P. de Kleijn, et al., Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome , Int. J. Proteomics 2012 (2012) 971907.
- T.S. Chen, R.C. Lai, M.M. Lee, A.B.H. Choo, C.N. Lee, S.K. Lim, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs , Nucleic Acids Res. 38 (2010) 215–224.
- R.W. Yeo, R.C. Lai, K.H. Tan, S.K. Lim, Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell, J. Circ. Biomark. 1 (2013) 7.
- R.C. Lai, R.W. Yeo, S.K. Lim, Mesenchymal stem cell exosomes, Semin. Cell Dev. Biol. 40 (2015) 82–88.
- B. Zhang, R.W. Yeo, K.H. Tan, S.K. Lim, Focus on extracellular vesicles: therapeutic potential of stem cell-derived extracellular vesicles , Int. J. Mol. Sci. 17 (2016) 174.
- Hu G-w, Q. Li, X. Niu, B. Hu, J. Liu, Zhou S-m, et al., Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice , Stem Cell Res. Ther. 6 (2015) 1–15.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li, et al., Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis , J. Transl. Med. 13 (2015) 1–14.
- B. Zhang, M. Wang, A. Gong, X. Zhang, X. Wu, Y. Zhu, et al., HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing, Stem Cells 33 (2015) 2158–2168.
- B. Zhang, Y. Yin, R.C. Lai, S.S. Tan, A.B.H. Choo, S.K. Lim, Mesenchymal stem cells secrete immunologically active exosomes , Stem Cells Dev. 23 (2013) 1233–1244.
- C.Y. Tan, R.C. Lai, W. Wong, Y.Y. Dan, S.-K. Lim, H.K. Ho, Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models , Stem Cell Res. Ther. 5 (2014) 1–14.
- C. Lee, S.A. Mitsialis, M. Aslam, S.H. Vitali, E. Vergadi, G. Konstantinou, et al., Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension , Circulation 126 (2012) 2601–2611.
- B. Yu, H. Shao, C. Su, Y. Jiang, X. Chen, L. Bai, et al., Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1 , Sci. Rep. 6 (2016) 34562.
- Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof of concept clinical trial. Stem Cells. 2014;32(5):1254–66.
- Vega, Aurelio, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
- Davatchi F, Sadeghi-Abdollahi B, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–5
- Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case- controlled study. Int Orthop. 2014;38(9):1811–1818
- Galli D, Vitale M, Vaccarezza M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:6.
- Beitzel K, Solovyova O, Cote MP, et al. The future role of mesenchymal Stem cells in The management of shoulder disorders . Arthroscopy. 2013;29(10):1702–1711.
- Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190.
- Malda, Jos, et al. " Extracellular vesicles [mdash] new tool for joint repair and regeneration. " Nature Reviews Rheumatology (2016).
Further References about PRP
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4(1):52–62.
Extras
- Xu, Ming, et al. " Transplanted senescent cells induce an osteoarthritis-like condition in mice. " The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (2016): glw154.
- McCulloch, Kendal, Gary J. Litherland, and Taranjit Singh Rai. " Cellular senescence in osteoarthritis pathology ." Aging Cell (2017).
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
Patient Services at ANOVA Institute for Regenerative Medicine
- Located in the center of Germany, quick access by car or train from anywhere in Europe
- Simple access worldwide, less than 20 minutes from Frankfurt Airport
- Individualized therapy with state-of-the-art stem cell products
- Individually planned diagnostic work-up which include world-class MRI and CT scans
- German high quality standard on safety and quality assurance
- Personal service with friendly, dedicated Patient Care Managers
- Scientific collaborations with academic institutions to assure you the latest regenerative medical programs