Promising New Treatment for COPD with Stem Cell Secretome
at ANOVA IRM in Offenbach, Germany
Chronic Obstructive Pulmonary Disease (COPD) is a group of progressive lung diseases, including emphysema and chronic bronchitis, that cause airflow blockage and significant breathing-related problems. COPD is characterized by chronic inflammation in the airways and lungs, leading to damage of the air sacs (alveoli) and thickening of the airway walls (see Fig. 1). This damage impairs the lungs' ability to exchange oxygen and carbon dioxide efficiently, leading to debilitating symptoms like shortness of breath. The severity of impairments varies among individuals, impacting their quality of life.
ANOVA Institute for Regenerative Medicine (IRM) utilizes Stem Cell Secretome, a cutting-edge, cell-free therapeutic derived from the paracrine factors of Mesenchymal Stem Cells (MSCs). The goal is to achieve potent anti-inflammatory, immunomodulatory, and regenerative effects within the damaged lung tissue. This innovative treatment can be delivered directly to the lungs via inhalation (aerosolized secretome) for targeted local action, or intravenously (IV) for systemic benefits. ANOVA IRM is among the pioneering clinics in Europe offering this advanced treatment for COPD.
Based on the known anti-inflammatory and regenerative properties of Mesenchymal Stem Cell secretome on lung tissue and the immune system, various stages of COPD can potentially be treated. This therapy aims to reduce chronic inflammation, protect lung cells, and support tissue repair, potentially improving lung function and quality of life for patients suffering from COPD.
To find out more about ANOVA's personalized Stem Cell Secretome treatment plans for COPD and our guidelines, schedule an appointment today.
COPD: Understanding the Disease
Diagnostics - Conventional Treatments - Advanced Stem Cell Therapies
On this page, we provide comprehensive information about Chronic Obstructive Pulmonary Disease (COPD), covering crucial aspects such as its causes, symptoms, current treatment options, and precision diagnostics. We also detail our innovative stem cell-based secretome therapies offered at our state-of-the-art facility in Offenbach (near Frankfurt am Main International Airport), Germany.
Jump directly to the following topics:
- Conventional COPD therapies
- ANOVA's advanced therapies for COPD
- Expectations, Potency, and Limits of Stem Cell Secretome
- Our Mesenchymal Stem Cell Secretome (MSEC) treatment options for COPD
- Workflow of the COPD treatment process
- FAQ - Frequently Asked Questions about COPD Stem Cell Therapy
- Scientific Sources and Literature
Conventional COPD Therapies: Current Standards of Care
Current treatments for Chronic Obstructive Pulmonary Disease (COPD) primarily focus on managing symptoms, reducing the frequency and severity of exacerbations (flare-ups), improving exercise capacity, and enhancing overall quality of life. Standard therapies include bronchodilators (to open airways), inhaled corticosteroids (to reduce airway inflammation), supplemental oxygen therapy for patients with severe COPD and low blood oxygen levels, and comprehensive pulmonary rehabilitation programs. While these interventions can significantly improve a patient's daily life and may slow disease progression, they do not reverse the underlying lung damage (emphysema or chronic bronchitis) characteristic of COPD. They predominantly address symptoms and complications rather than repairing the damaged lung tissue or fully halting the inflammatory cascade.
Many conventional medications carry potential side effects, and while pulmonary rehabilitation is highly beneficial, it demands considerable patient commitment and does not restore lost lung tissue or function. Consequently, there remains a significant unmet medical need for novel therapeutic approaches that can more effectively target the chronic inflammation, protect existing lung cells, and potentially stimulate regeneration of damaged lung tissue in COPD patients.
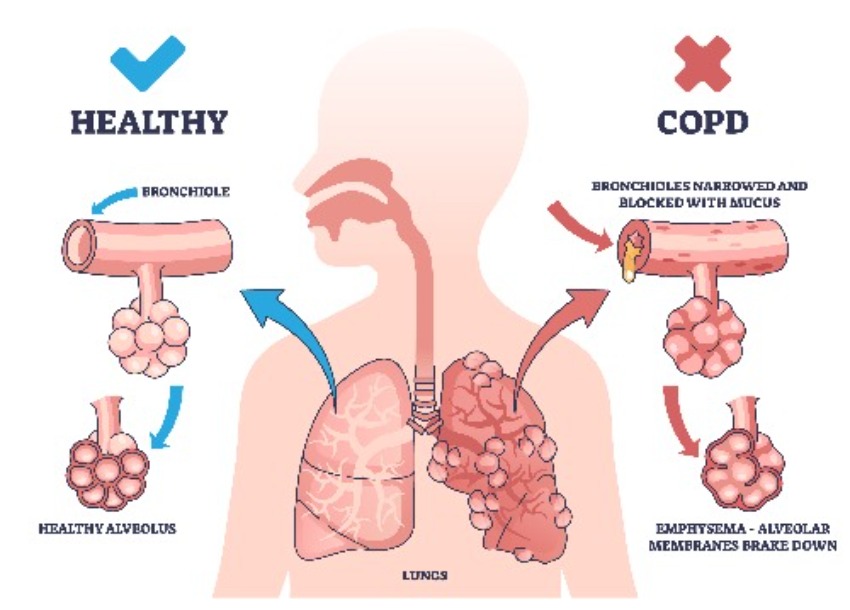
Fig. 1: Lung damage in COPD compared to a healthy lung.
ANOVA IRM - Germany
Stem Cell Secretome Therapies for COPD: A New Frontier
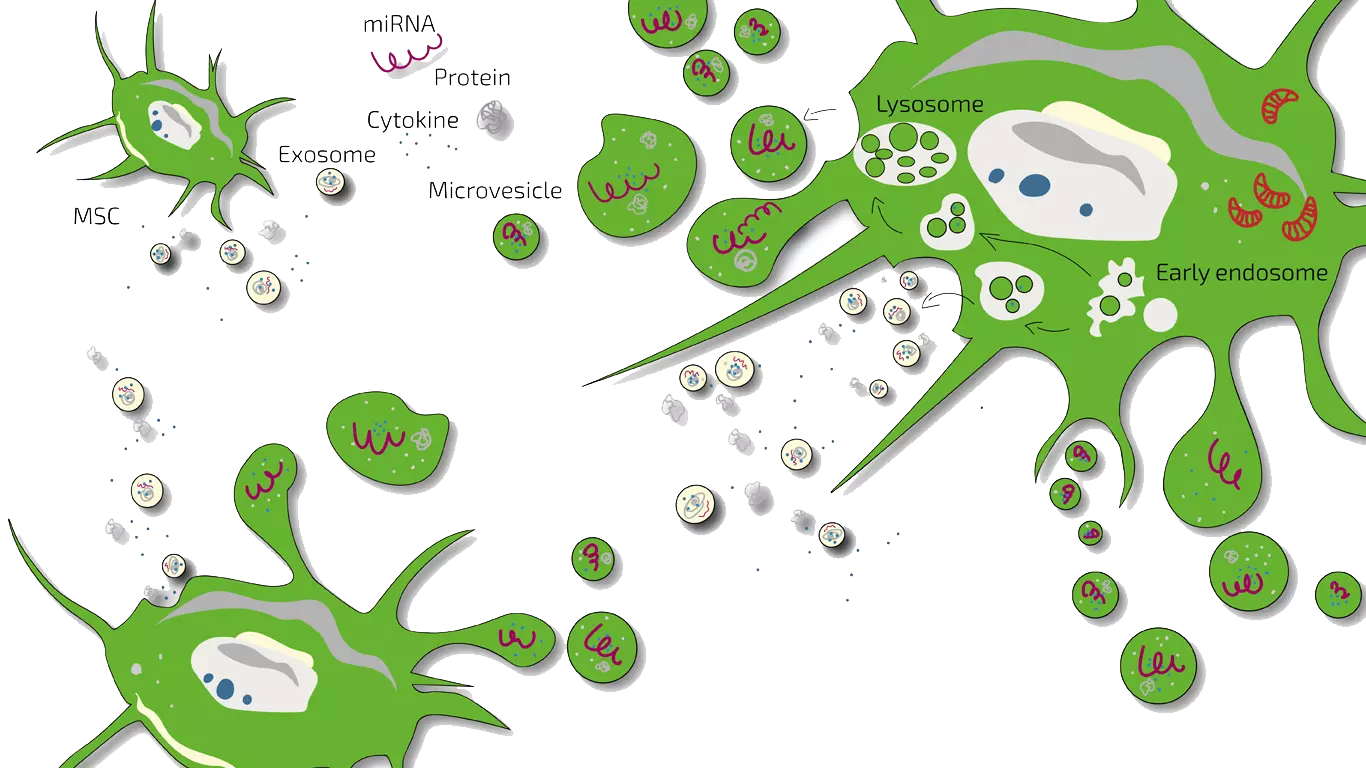
Stem cell-based therapies, particularly using Mesenchymal Stem Cells (MSCs) and their secreted products (the secretome), have emerged as a highly promising novel treatment avenue for COPD. MSCs are known for their potent immunomodulatory and anti-inflammatory properties, as well as their ability to stimulate tissue repair and regeneration. The MSC secretome, which is cell-free, contains a rich cocktail of bioactive molecules including cytokines, chemokines, growth factors, and extracellular vesicles like exosomes. These components work synergistically to reduce chronic inflammation, protect lung cells from further damage, and promote a healing environment within the lungs.
For COPD, MSC secretome therapy appears to offer two major beneficial effects:
- Immunomodulation and Anti-inflammation: Modulating the hyperactive immune response in the lungs, significantly reducing chronic inflammation that drives COPD progression.
- Tissue Protection and Regeneration: Stimulating the repair and regeneration of damaged lung tissue, potentially improving lung structure and function by promoting the survival of existing lung cells and supporting endogenous repair mechanisms.
Clinical trials and preclinical studies investigating MSCs and their secretome for COPD have shown encouraging results, including:
- Significant reduction in systemic and airway inflammation markers.
- Improvements in lung function parameters, such as Forced Expiratory Volume in 1 second (FEV1) and Forced Vital Capacity (FVC).
- Enhanced exercise tolerance and overall quality of life, as measured by tools like the 6-minute walk test and St. George's Respiratory Questionnaire (SGRQ).
- A reduction in the frequency and severity of COPD exacerbations.
Crucially, these therapies have generally demonstrated a good safety profile in clinical studies, with minimal side effects reported, especially when using autologous (patient-derived) products like the MSEC offered at ANOVA IRM.
Stem cell research advancements have enabled ANOVA IRM, a leading German Stem Cell Clinic near Frankfurt Airport, to offer this novel treatment: The ANOVA Stem Cell Secretome (MSEC). This cell-free therapeutic is a promising option for COPD management. It can be administered via inhalation (aerosol) for direct delivery to the affected lung tissues or intravenously for broader systemic anti-inflammatory and regenerative effects, tailored to the individual patient's needs. Contact us to learn more.
Check your eligibility for COPD stem cell secretome treatment, apply for a consultation, or simply write to us to receive more detailed information.
Stem Cell Secretome Treatments for COPD at
ANOVA Institute for Regenerative Medicine - Offenbach, Germany
Utilizing Autologous Mesenchymal Stem Cell (MSC) Secretome/Exosomes
Potency Hypothesis and Therapeutic Rationale of Stem Cell Secretome in COPD
Mesenchymal Stem Cells (MSCs) possess a remarkable ability to sense their microenvironment and respond by secreting a complex array of bioactive molecules collectively known as the secretome. In the context of COPD, the therapeutic potential of MSC secretome lies in its capacity to:
1. Modulate aberrant immune responses: The secretome contains anti-inflammatory cytokines (e.g., IL-10, TSG-6) and other factors that can dampen the chronic inflammation in the lungs, a hallmark of COPD.
2. Promote tissue repair and regeneration: Growth factors (e.g., KGF, HGF) and other signaling molecules within the secretome can stimulate the proliferation and differentiation of endogenous lung progenitor cells, protect existing cells from apoptosis (programmed cell death), and encourage remodeling of the damaged lung tissue.
3. Exert antimicrobial effects: Some components of the secretome may help combat infections, which are common triggers for COPD exacerbations.
Extracellular vesicles, particularly exosomes, within the secretome are key players, acting as natural nanocarriers to deliver proteins, lipids, and microRNAs to target cells, thereby mediating these therapeutic effects.
MSEC - Autologous Mesenchymal Stem Cell Secretome (Exosomes, EVs) for COPD
As COPD is a chronic, progressive, and currently incurable disease, ANOVA IRM focuses on treating patients in early to moderate stages with MSEC (Mesenchymal Stem Cell Secretome, rich in exosomes and other extracellular vesicles). This therapeutic is derived from the patient's own adipose-derived MSCs (AD-MSCs), harvested via a minimally invasive mini-liposuction procedure under light sedation. ANOVA IRM is one of the first clinics worldwide to obtain governmental authorization for producing and administering this high-quality, safe, and legally compliant autologous MSEC.
A significant advantage of MSEC is its cell-free nature. Unlike live stem cells, which can lose potency upon freezing, the MSEC can be cryopreserved without significant loss of its therapeutic components, including exosomes. This allows us to generate multiple (10-20) application doses from a single liposuction. These doses can then be administered over an extended treatment period, either via intravenous (IV) infusion for systemic effects or as an inhaled aerosol for direct lung delivery. This flexibility is particularly beneficial for the long-term management of COPD, enabling repeated immunomodulation and stimulation of lung cell survival and regeneration. For a detailed explanation of what Secretome/Exosomes are and their therapeutic advantages, please visit our overview page.
Please be aware that MSEC therapy for COPD is an experimental treatment and not a cure. It is designed as a potentially disease-modifying therapy. Successful treatment requires regular and repeated travel to our clinic in Offenbach, Germany, for applications.
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
Therapy Workflow for MSEC Treatment of Early to Mid-Stage COPD
The detailed workflow for our Secretome/Exosome therapy is outlined on its specific page.
All our therapies follow a structured process:
- Evaluation of medical history (analysis of current therapies, medical records, lung function tests like spirometry, imaging like CT scans).
- Initial consultation and assessment of potential patient-individual benefit from MSEC therapy (indication statement).
- Comprehensive preliminary examinations and advanced diagnostics.
- Detailed discussion of all therapy options, including potential benefits and risks.
- Creation of a personalized treatment plan, including a transparent cost estimate.
- Harvesting of adipose tissue (mini-liposuction).
- GMP-compliant production of the autologous MSEC product.
- Stringent quality control of the MSEC product.
- Application of MSEC according to the personalized plan (inhaled aerosol or IV infusion).
Eligibility: We primarily treat patients with early to mid-stage COPD. Patients must be able to breathe unassisted, be fit for light sedation, and undergo the mini-liposuction procedure. Children and pregnant women are not eligible. Other co-existing medical conditions might also be exclusion criteria, determined on a case-by-case risk-benefit assessment.
How Long Does the Stem Cell Secretome Therapy Process Take?
Initial analyses and remote consultations (phone/video conference) can take 2 weeks to a few months, depending on patient slot availability. For the tissue harvesting, a 2-day visit to Offenbach, Germany is required. MSEC production and quality control take approximately 4 weeks. Following this, the application phase begins according to your personalized treatment schedule, requiring regular visits to Offenbach for either inhalation or IV administration of the MSEC. The treatment pattern is adjusted to your travel capacity. The cryopreserved MSEC doses have a shelf life of 2 years.
Given that COPD is a chronic condition, we often recommend a "double-lipo" to produce enough MSEC doses (approx. 20) for a continuous treatment regimen over 2 years. A new liposuction would be needed thereafter if treatment is to be continued.
How Much Does Stem Cell Secretome Treatment for COPD Cost?
Our MSEC treatments are highly personalized. Costs vary based on the specifics of your condition, the number of MSEC doses required, the frequency of application, additional diagnostic needs, and sedation preferences. Treatment for COPD with MSEC typically costs well above ten thousand euros. A detailed, individualized cost estimate is provided after the initial assessment and before any treatment commitment.
Does Health Insurance Cover MSEC Therapy Costs for COPD?
Currently, experimental therapies like MSEC treatment for COPD are generally not covered by public or private health insurance. Patients are typically responsible for the full cost of the treatment.
Treating Early-to-Mid-Stage COPD with Advanced Regenerative Medicine at ANOVA IRM
The ANOVA IRM team is dedicated to providing patients with personalized regenerative medicine treatments for COPD. We integrate established best practices with innovative therapies like MSEC to strive for the best possible outcomes. A thorough diagnostic work-up, including pulmonary function tests (e.g., spirometry, DLCO), imaging (CT scans), and blood analyses, is essential to understand each patient's unique condition. This combination of advanced diagnostics, state-of-the-art MSEC production, and evidence-informed application protocols defines our specialized approach. However, it's crucial to understand that experimental therapies like MSEC treatment cannot guarantee success. Our physicians conduct a rigorous individual assessment to determine if the potential benefits of MSEC therapy outweigh the potential risks for each COPD patient. Make an appointment today to explore your personalized treatment options for COPD at ANOVA IRM.
Stem Cell Secretome Treatment for COPD: Personalized, High-Quality Care in Germany
ANOVA IRM leverages cutting-edge scientific research to produce and apply effective stem cell secretome therapies for COPD. Our clinic holds all necessary legal permits from German regulatory authorities for our therapeutics and undergoes bi-annual inspections. All MSEC products are manufactured under strict GMP-comparable conditions and undergo pharmaceutical-grade quality controls that often exceed legal requirements, ensuring patient safety and product quality.
We have implemented standardized operating procedures for MSC isolation, MSEC processing, and comprehensive diagnostic evaluations. This includes advanced pulmonary diagnostics (spirometry, body plethysmography, diffusion capacity testing), high-resolution CT imaging, and extensive blood biomarker analysis. We objectively track therapeutic progress after MSEC administration (via inhalation or IV) using these parameters. Our goal is to deliver the most advanced and personalized MSEC therapy for patients seeking innovative treatment for COPD in Germany.
Stem cell secretome therapy remains an experimental therapy. A thorough consultation with our specialist physicians is necessary to assess the individual risk-benefit profile. If deemed appropriate, MSEC therapy can be offered as a treatment option. To learn more about your COPD treatment possibilities at ANOVA Institute for Regenerative Medicine, a private German clinic specializing in advanced regenerative therapies, please click here to contact us.
FAQ: Stem Cell Secretome Therapies for COPD
What is COPD?
Chronic Obstructive Pulmonary Disease (COPD) is a progressive, chronic inflammatory lung disease that obstructs airflow from the lungs, making breathing difficult. It encompasses conditions like emphysema (damage to air sacs) and chronic bronchitis (long-term inflammation of bronchial tubes). COPD typically worsens over time and is a major cause of disability and mortality worldwide. While current treatments manage symptoms, there is no cure.
What are the Common Symptoms of COPD?
COPD symptoms often develop gradually and may not be noticeable until significant lung damage has occurred. Common symptoms include:
- Persistent shortness of breath (dyspnea), especially during physical activity.
- Chronic cough, often with mucus (sputum) production.
- Wheezing and chest tightness.
- Frequent respiratory infections.
- Fatigue or lack of energy.
- In later stages, unintended weight loss and swelling in ankles, feet, or legs.
COPD patients may also experience exacerbations (flare-ups) where symptoms worsen acutely.
How is Stem Cell Secretome Administered for COPD at ANOVA IRM?
At ANOVA IRM, the Mesenchymal Stem Cell Secretome (MSEC) for COPD can be administered in two primary ways, based on the patient's individual needs and treatment plan:
1. Inhalation (Aerosol): The MSEC is nebulized into a fine mist that the patient inhales directly into the lungs. This allows for targeted delivery of the therapeutic factors to the site of inflammation and damage.
2. Intravenous (IV) Infusion: The MSEC is administered directly into the bloodstream. This route allows for systemic distribution of the secretome's components, which can be beneficial for addressing widespread inflammation and potentially exerting effects beyond the lungs.
The choice of administration route is determined by our specialists during the treatment planning phase.
Is Stem Cell Secretome Therapy a Cure for COPD?
No, Stem Cell Secretome therapy is not considered a cure for COPD. COPD is a chronic and complex disease. MSEC therapy is an experimental treatment aimed at managing symptoms, reducing inflammation, potentially slowing disease progression, and improving quality of life. It is a disease-modifying approach, not a curative one. Realistic expectations are important, and our team will discuss potential outcomes thoroughly.
What are the Potential Benefits and Risks of Stem Cell (MSEC) Therapy for COPD?
Potential Benefits: Based on ongoing research, potential benefits may include reduced airway inflammation, improved lung function parameters (e.g., FEV1), decreased frequency of exacerbations, enhanced exercise capacity, and better overall quality of life.
Potential Risks: Since we use autologous (patient's own) MSEC, the risk of immune rejection or transmission of infectious diseases is virtually eliminated. The mini-liposuction procedure carries minimal risks typical of minor surgical interventions (e.g., bruising, local discomfort). The administration (inhalation or IV) is generally well-tolerated. As an experimental therapy, long-term effects are still under investigation. All potential risks are discussed in detail during the consultation.
What Causes COPD and What are the Main Risk Factors?
The leading cause of COPD in developed countries is long-term tobacco smoking. In developing nations, exposure to fumes from burning fuel for cooking and heating in poorly ventilated homes is also a major contributor. Other factors include long-term occupational exposure to dust, chemicals, and air pollution.
These irritants trigger chronic inflammation in the lungs, leading to progressive damage to the airways and air sacs (alveoli), resulting in airflow limitation and breathing difficulties.
Key Risk Factors for Developing COPD:
- Tobacco Smoking: The most significant risk factor.
- Exposure to Secondhand Smoke.
- Occupational Dusts and Chemicals: Long-term exposure in certain industries.
- Air Pollution: Both indoor (e.g., biomass fuel) and outdoor pollution.
- Genetics: Alpha-1-antitrypsin deficiency is a rare genetic cause. Other genetic predispositions may exist.
- Age: COPD typically develops in individuals over 40.
- History of Childhood Respiratory Infections or Asthma.
Understanding COPD: Components, Disease Course, and Complications
COPD typically involves a combination of:
- Chronic Bronchitis: Persistent inflammation and narrowing of the bronchial tubes, leading to chronic cough and mucus.
- Emphysema: Destruction of the alveoli (air sacs), reducing the surface area for gas exchange and causing air trapping.
The disease is generally progressive, with lung function declining over time, especially if exposure to irritants continues. COPD severity is often staged (e.g., GOLD stages 1-4) based on spirometry results. Exacerbations can accelerate disease progression.
Common complications of COPD include:
- Frequent respiratory infections (pneumonia, bronchitis).
- Pulmonary hypertension and heart problems (cor pulmonale).
- Increased risk of lung cancer.
- Depression, anxiety, and social isolation.
- Malnutrition and muscle wasting.
The Scientific Rationale for Using Stem Cell Secretome in COPD Treatment
"COPD is driven by chronic inflammation and progressive lung tissue destruction. Stem cell secretome therapy aims to counteract these processes by delivering a potent mix of anti-inflammatory and regenerative molecules directly or systemically."
Mesenchymal Stem Cells (MSCs) are multipotent stromal cells that do not function by differentiating into lung cells, but rather by secreting a wide range of bioactive molecules – the "secretome." This secretome is rich in paracrine factors such as anti-inflammatory cytokines (e.g., IL-10, TSG-6), growth factors (e.g., Hepatocyte Growth Factor - HGF, Keratinocyte Growth Factor - KGF), and extracellular vesicles, most notably exosomes. Exosomes are nano-sized vesicles that shuttle proteins, lipids, and microRNAs between cells, acting as key mediators of intercellular communication and therapeutic effects.
In COPD, the MSC secretome is hypothesized to:
- Reduce chronic inflammation by modulating immune cell activity.
- Protect lung epithelial and endothelial cells from apoptosis (cell death).
- Stimulate proliferation and differentiation of endogenous lung progenitor cells.
- Promote angiogenesis (new blood vessel formation) where needed.
- Reduce fibrosis (scarring) in the airways.
Emerging scientific data continues to support the potential benefits of MSC-derived secretome/exosome therapies for chronic inflammatory and degenerative lung diseases like COPD. For more detailed information on current research, please contact us.
Scientists and physicians recognize the significant potential of MSC secretome to ameliorate COPD symptoms and slow disease progression. While not a cure, this approach may offer a valuable addition to current COPD management strategies. Further clinical research is essential to fully establish its efficacy and long-term benefits.
If you have questions regarding our MSEC treatments for COPD, please contact us or apply for a treatment consultation here.
References and Literature - Stem Cell-based Therapies for COPD
Note: This is a representative list. A comprehensive, up-to-date list of scientific publications supporting our specific MSEC therapy can be provided upon request during consultation.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). (General COPD guidelines)
- Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590-1598. (Example of MSC clinical trial)
- Broekman W, Khedoe P, Schepers K, et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Airway Inflammation and Remodeling in a Murine Model of Chronic Obstructive Pulmonary Disease. Cells. 2021;10(9):2209. (Example of EV/exosome preclinical study for COPD)
- Kim YS, Kim JY, Huh JW, et al. The impact of human umbilical cord blood-derived mesenchymal stem cells in patients with moderate-to-severe chronic obstructive pulmonary disease: a pilot study. Korean J Intern Med. 2021;36(3):588-597. (Example of another MSC source study)
- Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22(6):824-833. (Review on MSCs)
- Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35(4):851-858. (Review on Exosomes)
- Harrell CR, Sadikot R, Pascual J, et al. Mesenchymal Stem Cell-Based Therapy for COVID-19 and Other Inflammatory Lung Diseases: A Promising Therapeutic Option. J Clin Med. 2021;10(10):2049. (Broader context of MSCs in lung disease)
Please contact ANOVA IRM for more specific literature related to our autologous MSEC therapy for COPD.
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)