Spinal Cord Injury - SCI:
An Innovative Treatment with Stem Cells and Robotic Exoskeletons
New Horizon: Combine Robotics and ANOVA IRM Stem Cell-based Therapy
The spinal cord is a column of nerve tissue that connects the brain with peripheral nerves. Damage to the spinal cord, by trauma or other means, consequently results in severe motor- and sensory deficits that usually lead to the inability to move and feel. Accidents are the most common cause of Spinal Cord Injury (SCI), with catastrophic consequences for the life of the patient and their relatives. While conservative therapies aim to stabilize the patient, functional recovery in most cases is minimal.
Now, there is new hope for patients with paralysis from spinal cord injury. The combination of mesenchymal stem-cell based neuro-regeneration with neuro-functional robotic exoskeleton training - the REMCell treatment - offers hope to get out of the wheelchair and walk again.
This is made possible by a unique collaboration between ANOVA Institute for Regenerative Medicine in Offenbach, Germany and Cyberdyne, a Japanese company with a training center in Bochum, Germany.
Since 2019 we co-operate with Cyberdyne Care Robotics
Whilst ANOVA is the first institution worldwide to have obtained a permit for the production of and treatment with mesenchymal stem cell secretome by the legal authorities,
Cyberdyne has developed the HAL exoskeleton for neuro-functional feedback training.
The REMCell treatment, the first combination of neuro-regeneration with stem cells and the neuro-functional training with the HAL exoskeleton, promises to get many paralyzed patients out of their wheelchair - and takes neuro-rehabilitation to the 21st century.
On this page we inform you about spinal cord injury (complete and incomplete, partial spinal cord injuries) covering an overview on important aspects of treatment options, precision diagnostics that reveals the cause of pain and location of the pain-causing defect, as well as our stem cell-based therapies that we offer in Offenbach (near Frankfurt/Main) Germany and the HAL exoskeleton therapy we recommend for SCI.
Jump directly to the following topics:
- Conventional therapies
- ANOVA therapies for spinal cord injury
- Expectations and limits
- Our spinal cord injury treatment with MSEC
- Recommended exoskeleton training program
- Workflow of the treatment process with stem cells only
- Workflow of the treatment process with REMCell
- Diagnostics of pain-causing defects
- The ANOVA difference: targeted treatment
- You want a second opinion
- Sources and Literature
We treat the following injuries and conditions:.
- Partial spinal cord injuries (cervical, thoracic and lumbar)
- Incomplete spinal cord injuries (cervical, thoracic and lumbar)
- Spinal cord compression (cervical, thoracic and lumbar)
- Spinal cord edema after surgery (cervical, thoracic and lumbar)
- Complete spinal cord injury in some cases (responsive, cervical, thoracic and lumbar)
Generally Available Conventional Therapies
Are Current Concepts of Neuro-Rehabilitation Outdated?
So far it was assumed that after spinal cord injuries the patients undergo three critical phases: The acute, sub-acute and chronic phase. During the chronic phase, beginning one year after the SCI, it was assumed that further functional recovery is not possible. It was also assumed that the main problem in patients with SCI is at the level of the injured spinal cord. Both assumptions have now been proven wrong, at least partially.
The misconceptions and lack of understanding of the real patho-mechanisms of SCI are responsible for the ineffectiveness of most currently used neuro-rehabilitation treatments, which are mainly aimed at stabilizing the patient's condition but not at the re-establishment of function. This pertains mainly to the sub-acute and chronic phase.
Unfortunately, even acute phase treatment does not always take into account the patho-physiology of SCI. In cases of local damage, e.g. by a fractured vertebra causing mechanical damage, the secondary damage caused by oedematous swelling of the cord, might significantly worsen the outcome. Spinal cord damage leads to bleeding and inflammation with the resulting accumulation of fluid in the cord (haematoma and oedema). This makes the spinal cord swell up. In a confined space such as the spinal canal the swollen cord cannot expand sufficiently. The swelling then leads to an increase of pressure in the cord, which compresses blood vessels and leads to a lack of blood supply, significantly enlarging the zone of damage. Early aggressive treatment, both surgical and with medication can limit this secondary damage reducing swelling and controlling blood pressure. If not applied properly this can lead to unnecessary additional loss of function for the patient.
Both external pre-clinical and external clinical studies have shown improved recovery of SCI patients when the therapy was combined with a suitable stem cell therapyxi. With ANOVA’s Stem Cell Secretome we provide access to the most advanced clinically available combination of authority-controlled stem cell-based therapies for the treatment of SCI.
Stem Cell Treatments for Spinal Cord Injury at
ANOVA Institute for Regenerative Medicine - Offenbach, Germany
BMC, Secretome/Exosomes, PRP
What Therapeutic Successes can be Expected?
Whilst HAL exoskeleton training by itself has already been shown to be much more effective than conventional neuro-rehabilitation training, the combination with stem cell-based neuro-regeneration promises even better results. External pre-clinical studies of the effect of mesenchymal stem cells and their secretome have shown numerous beneficial effects on the damaged spinal cord tissue, such as the re-organization of glial scars, improved vascularization and the promotion of axonal growth.
As for the HAL exoskeleton, research has shown that neuro-functional training improves the patients' independence, especially their ability to walk, significantly. Walking with a rollator, crutches or without crutches became possible for many patients previously confined to the wheelchair.
But there are also other benefits: Patients experienced a decrease in neuro-pathic pain, positive changes in spasticity, improved sensitivity and, as a result, a reduced risk of pressure sores. After completion of neuro-muscular feedback therapy, the successes achieved are maintained as long as the patients actively use their regained mobility in everyday life, i.e. getting out of the wheelchair and walking with a rollator or using crutches.
Potency Hypothesis of Stem Cell Therapies
Stem cells possess the potential to communicate with immune cells that elicit the inflammation and by natural, so far not understood mechanisms may inhibit this immune-over-reaction. After halting the inflammatory reaction in damaged areas, stem cells have the ability to stimulate regeneration of tissue. Negative long-term effects similar to cortisone are not to be expected. The aim of a stem cell treatment is therefore, the fast relief of pain, the inhibition of inflammation and in the best cases to even support regeneration. The effect on regeneration has the highest chance of success when combined with intense, modern types of physiotherapy such as the HAL exoskeleton program. This can dramatically increase the quality of life, especially for patients with severe pain, as well as the movement duration and range.
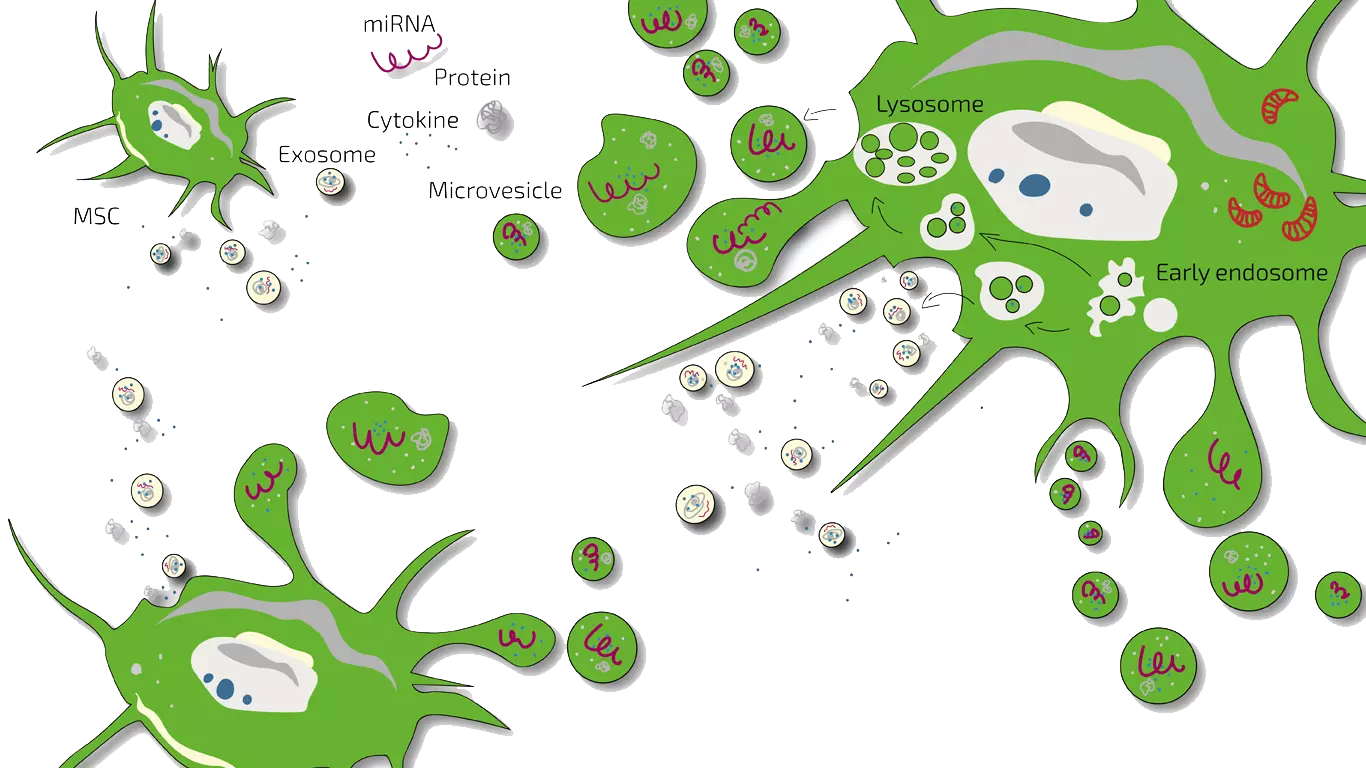
MSEC - Mesenchymal Stem Cell Secretome - Exosomes - Autologous
Depending on the type of SCI we either treat patients with MSEC or BMC or a combination of both. MSEC (secretome, exosomes, EVs) of mesenchymal stem cells (MSC, AD-MSC, adipose-derived, fat-derived stem cells) which we harvest from the patients belly in a mini-liposuction (very brief and limited liposuction) under slight sedation is given intravenously and is meant to act more systemically. Worldwide, ANOVA is the first stem cell clinic to acquire legal permission from the responsible governmental authorities and therefore, offers high quality, safe and legally-controlled autologous (own) exosome-containing secretome.
The main advantage of MSEC is that it can be frozen without loss of exosomes which is in contrast to live stem cells which would lose their therapeutic potency. This enables us to produce 10-20 injection doses from one liposuction which can then be administered over a longer treatment period. This is especially advantageous for SCI as the regenerative . The definition and function of Secretome/Exosome is explained on our overview page. Please note that this treatment should be combined with HAL training below and requires repeated travelling to Offenbach, Germany.
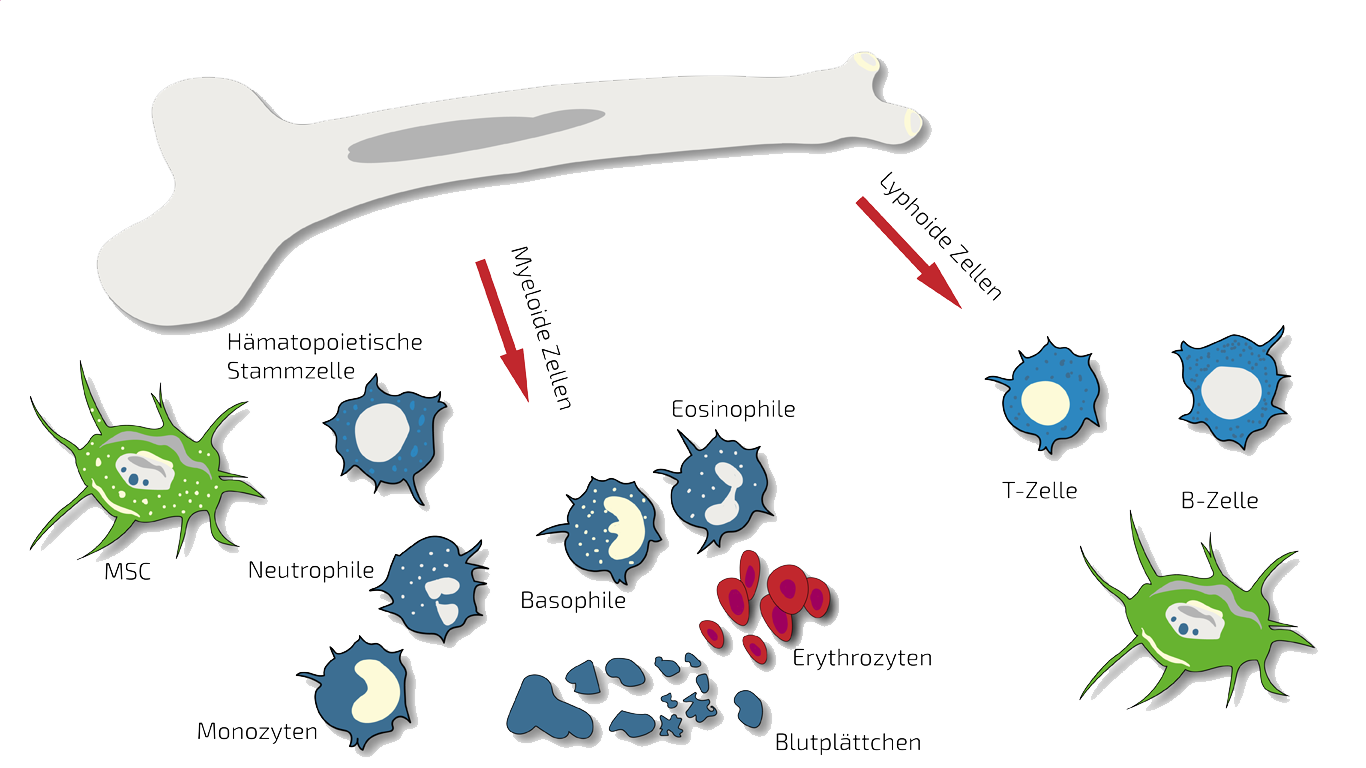
BMC - Bone Marrow Concentrate - Autologous
Autologous (self) BMC is the type of therapy we use for locally-restricted damage in SCI especially when only one vertebra is affected.
In such cases we treat specifically this region with targeted, localized BMC injections. BMC contains autologous, adult stem cells (hematopoietic and mesenchymal stem cells in natural composition) which we isolate and concentrate from the patients pelvis crest in a short process under slight sedation.
These stem cells are supposed to inhibit the inflammation thereby relieving you from pain and to stimulate regeneration of the damaged bone or cartilage. For an on-going therapy, we combine BMC with MSEC above. More information about this type of stem cell therapy can be found here: BMC.
HAL® - Hybrid Assistive Limb - Robotics - Exoskeleton
Since 2019 we co-operate with Cyberdyne Care Robotics.
This Japanese company with branches in Germany (e.g. Bochum) offers technically state-of-the-art rehabilitation procedures and programs with exoskeletons (HAL®, Hybrid Assistive Limb) and functional training for spinal cord injury patients that show improvement after physio therapy (incomplete SCI, responsive SCI).
Good news is that Cyberdyne has reached another mile stone towards having statutory health insurance to reimburse cost of HAL therapy for patients.
Paraplegics are treated with great success in 3-month training programs if nerve signals are measurable in an evaluative first session. Patients usually achieve a significant improvement in their ability to move, even to the point of walking on a rollator or walker. Patients stay in Bochum, Germany for 3 months and train 5 days a week. One session is approximately 500 Euro.
Many patients combine our MSEC or BMC treatment with these nerve-guided exoskeleton robotics programs to further increase the chances of success.
We will be happy to advise you on this as well! Or read more in either the following sections or our latest flyer.

HAL Exoskeleton-Training
ANOVA IRM - © Cyberdyne
PRP - Platelet-Rich Plasma - Autologous
PRP is a comparably inexpensive experimental therapy as platelets (thrombocytes naturally containing growth factors and stimulants) are isolated from autologous (own) blood without isolation of stem cells.
For spinal cord injury, we use PRP only in combination with BMC or MSEC. It is administered in-between BMC treatments to continuously support the anti-inflammatory and growth promoting effect.
Besides this, PRP is well-known as a stimulant for wound healing in e.g. periodontitis therapy or as a measure against hair loss. More on PRP (as a combination therapy) is summarized on our PRP overview page.
Hyaluronic Acid - HA
We only rarely treat spinal cord injury patients with hyaluronic acid, as HA has no regenerative potential in itself.
In cases of intervertebral disc damage in parallel to SCI, we generally would treat patients either with BMC or MSEC for their greater potential.
Hyaluronic acid - HA
ANOVA IRM - Germany
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
Therapy Workflow for Stem Cell Treatment of Spinal Cord Injury - SCI
The precise workflow is described in detail on the stem cell- specific pages of BMC , Secretome/Exosomes and PRP (as combination therapy).
All therapies are divided into phases such as evaluation of your medical history (we analyze your current therapies and medical records), initial counseling and evaluation of potential therapy outcome for your specific situation, Patient-individual benefits of a stem cell therapy (indication statement) will be discussed as well, preliminary examinations, diagnostics, consultation on all therapy options. Finally, the preparation of an individual treatment plan including cost estimate, harvesting of tissue, production and quality control of the stem cell product and application will be planned.
In addition, we often apply the stem cells (BMC) intra-articularly (i.e., directly in the joint). This means that we deliver the stem cells to the exact location where your pain originates.
Unfortunately, according to the risk-benefit ratio, we cannot treat children or pregnant women. In addition, other factors can also be exclusion criteria.
How Long Does a Stem Cell Therapy Take?
The initial analyses and counseling can be done without you having to travel to Offenbach (near Frankfurt/Main, Germany). This period can be 2 weeks up to months depending on the availability of patient slots. If you live further away, we will conduct the initial discussions by telephone or video conference. For the actual treatment, you will have to travel to Offenbach. Then, depending on the therapy procedure, the tissue collection, quality control and treatment will approximately follows these time lines:
BMC- and PRP Therapy
Each donation and application of BMC and PRP (as combination therapy) on-site period: 2 days (consecutive days).
Secretome/Exosome Treatment:
For harvest and preparation and harvest of the fat (mini-liposuction) 2 days (consecutive) are required in Offenbach. Enrichment of the mesenchymal stem cells (secretome/exosomes) and the respective quality control will follow afterwards.
Approximately 4 weeks after the isolation, the therapy can begin according to the therapy plan determined with you. You will then come back to Offenbach am Main (Germany) several times for the application. The shelf life of the secretome (exosomes) is approximately 2 years.
How Much Does Stem Cell Treatment for Spinal Cord Injury Cost?
Our treatments are always tailored to your specific situation, disease, stage and other factors. The therapies differ in the product used (BMC, secretome, PRP or hyaluronic acid), the frequency of treatment as well as the further examinations and your sedation and anesthesia wishes. A treatment for spinal cord injury will cost several ten-thousand euros when combined with HAL training. You will receive a cost estimate for all treatments in advance so that you can accurately estimate what a treatment would cost in your individual case.
Does my Health Insurance Cover the Therapy Costs?
Unfortunately, at the moment it is assumed that health insurance companies do not cover the costs of experimental therapies (BMC, secretome, PRP), i.e. you will have to bear the costs entirely yourself.
Workflow Stem Cells and Robotic Exoskeletons: REMCell Therapy –
A Novel Approach to Neuro-Rehabilitation
ANOVA Institute for Regenerative Medicine, Germany’s pioneering institution for stem cell therapies, and Cyberdyne, the Japanese pioneer in robotic neuro-functional training, have formed an alliance to establish a novel and effective treatment for spinal cord injury: REMCell, Robotic Exoskeleton and Mesenchymal Stem Cell Therapy. REMCell integrates neuro-regeneration (NR) with stem cells and neuro-functional (NF) training with the HAL robotic exoskeleton, they have established what currently constitutes the most promising and probably the most effective way for functional recovery after a spinal cord injury.
Therapy Workflow: A complete Course of REMCell Therapy Takes 3 Months
Phase 1 - Evaluation of Suitability for REMCell Therapy
The patients are first evaluated for remaining nerve signals in their legs and whether they are a suitable donor for mesenchymal stem cells. If these tests are positive, the REMCell Therapy can be commenced.
Phase one takes a day or two for the patient, and about a week for the results to return from the laboratories.
Phase 2 - Stem Cell Harvesting and Secretome Production
Stem cell therapy requires the harvesting of mesenchymal stem cells (MSC) from the subcutaneous fat, which is done with a mini-liposuction, similar to cosmetic liposuction. This is a minimally invasive procedure and takes approximately 1 hour in the OR, and altogether half a day for the patient.
The stem cells are then isolated from the fat and grown in the laboratory. When a sufficient number of stem cells has been grown, the cells are put under certain conditions, which will optimize the secretome production by the cells. 10 or 20 doses of MSC secretome can be produced and stored for a maximum of two years. The production takes 4 weeks to complete.
Phase 3 - Neuro-Functional Training and Neuro-Regenerative Stem Cell Treatment
Immediately after the stem cell harvesting has been completed, the patient starts with HAL training, 5 days a week with a daily session lasting 1 - 2 hours each. Weekends are free.
After 4 weeks of training, the MSC secretome production is complete and the first infusion of MSC secretome is carried out. Alternative to the intravenous infusion, the MSC secretome can also be injected intrathecally, i.e. into the cerebro-spinal fluid (CSF) surrounding the spinal cord and brain. Depending on whether 10 or 20 doses of MSC secretome were produced, the patient receives infusions/injections every two weeks or weekly.
How can you Participate in ANOVA's and Cyberdyne's
Combined Neuro-Regenerative and Neuro-Functional Therapy?
For neuro-muscular feedback therapy in paraplegic patients, weak residual neurological impulses in the musculature must be present and strong enough to be recorded. Other patient groups, stroke patients and neuro-muscular patients, must also meet appropriate eligibility requirements. For the stem cell production, donor suitability must be established.
In order to find out whether you can profit from ANOVA’s and Cyberdyne’s combined stem cell and HAL training therapy, talk to our patient care managers. We will evaluate your medical records and assess you on-site.
Patient Testimonial After HAL Therapy
Gianna Regenbrecht
"To see yourself walking again, that's really touching!"
Since I started training with HAL in November 2014, I've come a giant step closer to my dream of being able to manage my daily life on my own feet again. I have feeling in my legs again and my muscles have gotten much stronger. In everyday life, this means that I can walk short distances with a rollator or crutches, I can stand up to reach higher things, I can climb steps with a little help, and I can put my wheelchair in the trunk and get into the car on foot. For me, this means a more self-determined everyday life and a great deal more self-confidence.
How does the ANOVA Therapy differ?
Diagnostics – We look for the Cause of your Pain
Dr. mult. Michael K. Stehling, the founder of ANOVA IRM and the Vitus Prostate Center , is a radiologist (MD) and holds a PhD in physics. For this reason, the ANOVA Institute for Regenerative Medicine, in cooperation with the Prof. Stehling Institute for Diagnostic Imaging located in the same building, has the capability to use special precision diagnostics such as arthro-MRI and non-radioactive contrast MRIs.
Compared to many conventional MRIs, these methods are often able to localize the pain-causing inflammation, degeneration and damage in your spine and vertebrae. This enables us to determine individually how patients should be treated and where the stem cells should be applied.
Furthermore, in consultation with you and if necessary or advisable, we supplement our patient-specific diagnostics with specific blood tests on hormones, inflammation parameters and other factors that are important in your case, or recommend further examinations such as a preventive MRI spinal scan.
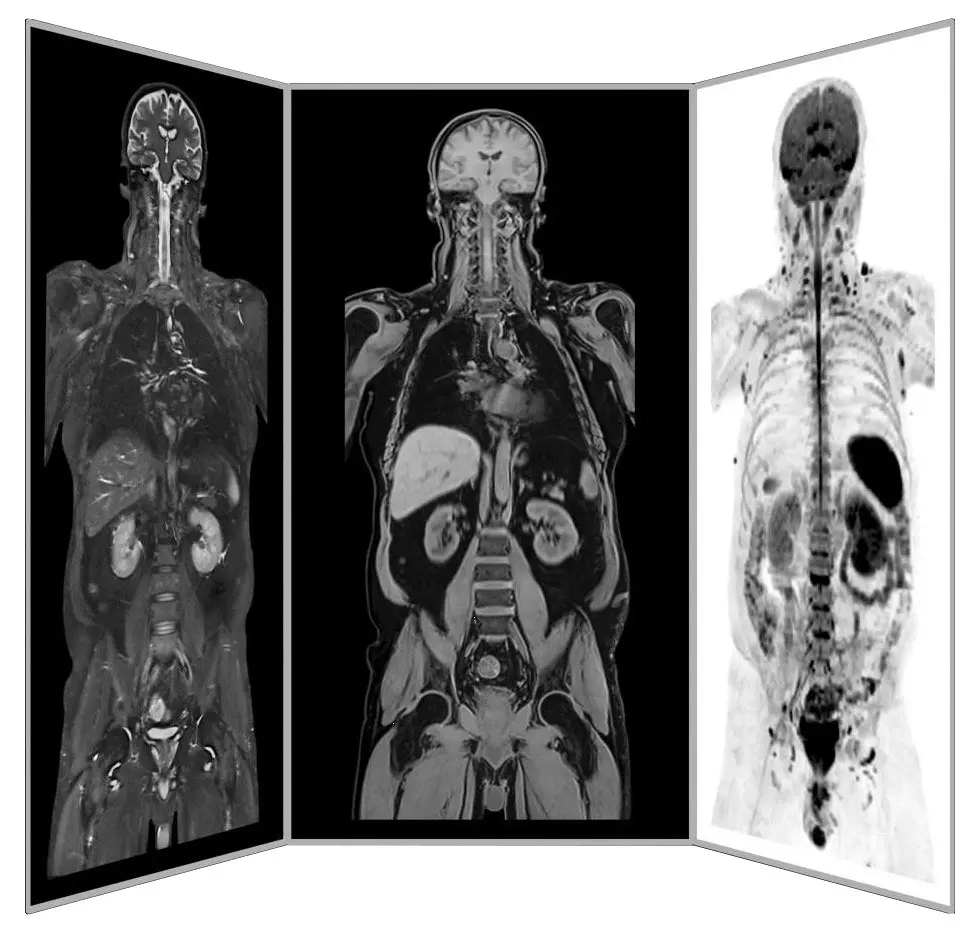
Precision MRI scans - find the source of pain
ANOVA IRM © Siemens Healthcare GmbH
How Does the ANOVA Therapy Differ?
We Implant the Stem Cells Precisely Where They are Needed
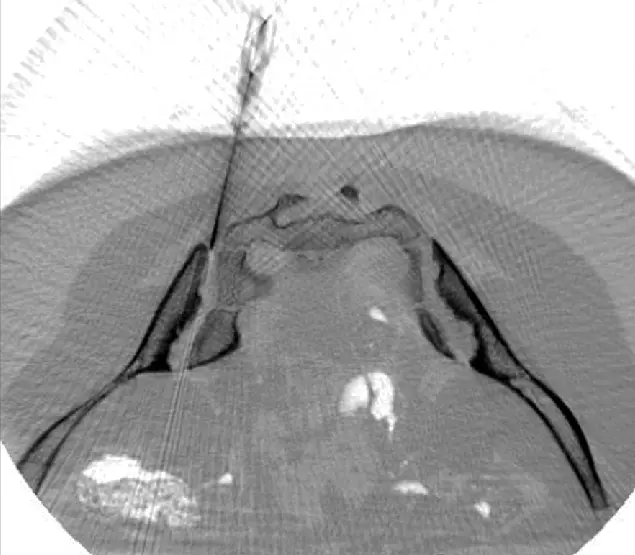
CT-assisted stem cell injection into joints
ANOVA IRM © Siemens Healthcare GmbH
Based on our specific diagnostics using arthro-MRI and non-radioactive contrast medium MRIs, we can, in contrast to many other clinics, deliver the stem cells with image support, e.g. using CT, precisely to the affected area. This means we can inject into and at joints, vertebrae and ligaments to specifically and quickly trigger an effect where inflammation causes pain. All interventions are performed under supervision and care of our anesthesiologist and are pain free.
A purely intravenous administration, as many other clinics do, is only performed for the secretome (exosomes) if this is to be used to treat chronic inflammatory conditions of the knee.
Of course, we will thoroughly advise you in the early process and the on-site consultation in advance on all steps and discuss alternatives and expectations.
Are you Interested but Uncertain? Book a Counselling Appointment!
Our patient care managers are happy to inform you about what information we need upfront (MRI, CT, X-ray), how to transfer large data files and schedule a counseling appointment with our physicians for you. Our patient care managers are happy to inform you about what information we need upfront, how to transfer large data files and schedule a counseling appointment with our physicians for you. Please use our contact form to support a fast processing of your case and request.
You are also always welcome to send us an e-mail about your case. The counseling appointment may also take place per telephone or video chat if you live outside Germany. For more intense counseling or additional diagnostic evaluations you may also book an on-site appointment. We can perform needed MRI on the same day. All services rendered by our patient care team are free of charge and we inform you about all physician appointment charges up-front.
Neuro-Functional Training With the HAL Robotic Exoskeleton
After a SCI, nerve signals from the brain to the muscles in the legs (efferent signals) might be to week to make muscles move. But in many patients these nerve signals are still recordable with sensitive electrodes. With the HAL robotic exoskeleton the weak nerve signals are amplified and used to activate electrical motors, which in turn move the patient’s legs, in an almost natural way.
This generates nerve signals in so-called proprioceptors in the muscles and joints, which are fed back to the brain (afferent signals). These afferent signals originating in the legs and going to the brain, and efferent singals, running down the spinal cord to the peripheral nerves form a feed-back loop. In patients with SCI this feedback loop is interrupted and with time degenerates.
Functional MRI studies, which can visualize brain activity, have shown that the areas in the motor cortex of the brain (gyrus praecentralis), which control specific movements of the legs and are normally very focused on small areas of the motor cortex, "smear out" over larger areas in patients with SCI. This might impede voluntary initiation of motion by deeper functional centers of the brain.
During HAL training, the functional activity is re-focused onto the original smaller areas. It thus appears that the re-establishment of the feed-back loop, particularly the sensory input from the proprioceptors in the leg to the brain, is an essential component of voluntary motion.
Hope for Patients with SCI - Scientific Results With Stem Cells
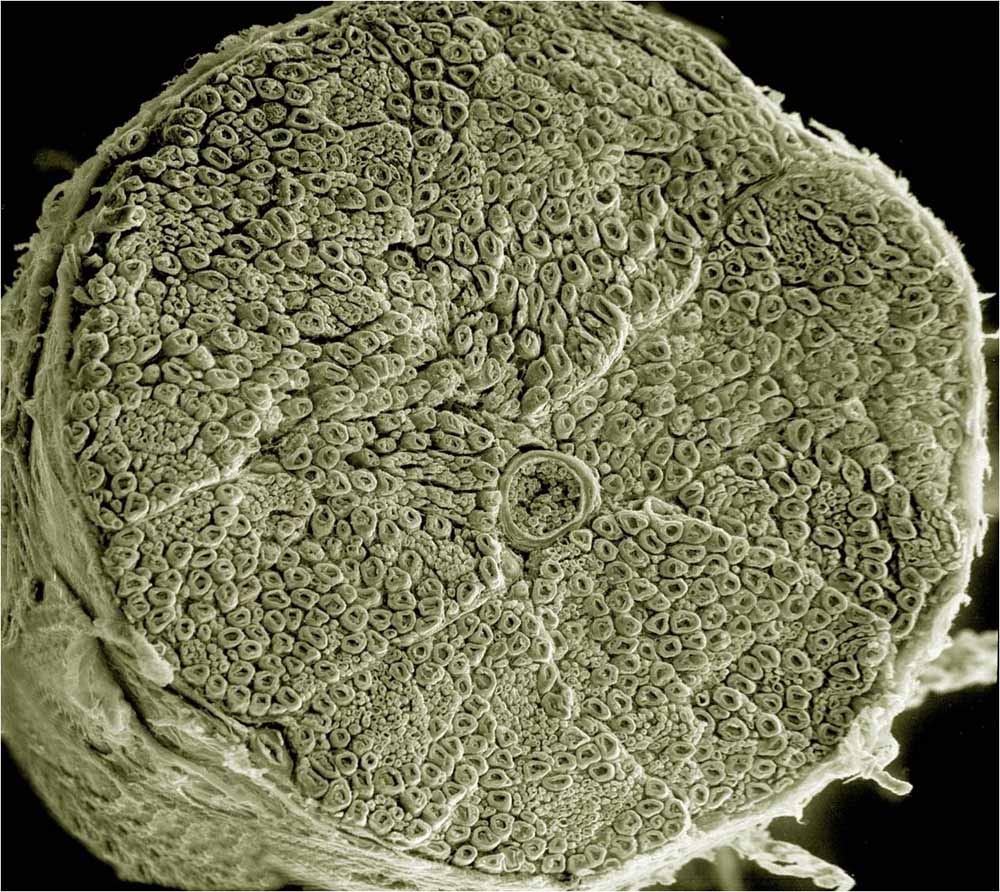
An electron microscopy image of a cross-section of a spinal nerve, illustrating the complexity on a tiny scale. This is why nerve repair needs to happen on the cellular level with stem cells, as it cannot be repaired in any other way.
Image from UCSD tumblr
Spinal trauma can disrupt ascending and descending axonal pathways that lead to inflammation, demyelination and loss of neural cells (neurons). Depending on the site of injury, functional disorders induced by cellular damage usually results in the inability to move, sensory loss and/or lack of autonomous nervous system control.
Fully regenerative therapies for spinal trauma do not exist yet. However, very promising results have been obtained with stem cell transplantation in patients with spinal trauma. The use of Mesenchymal Stem Cells (MSCs) in Spinal Cord Injury has been extensively reviewed. Experiments with MSCs have shown that their abilities to stimulate repair processes in spinal cord injury are due to the paracrine secretion of bioactive compounds by the MSCs. After 21 days of observation, there was a significant improvement in functional recovery, even though the MSCs had not been incorporated into the regenerated host tissue, from as early as one week after the treatment with MSCs.
The progress in this area has recently been reviewed in the scientific paper from Lamichhane and colleagues.
There is no treatment (experimental or established) for which your treating physician can promise or even guarantee a therapeutic success. In the case of stem cell therapy, which is an experimental treatment, doctors are obliged to analyze the benefits and risks for each individual case and ensure that the benefits of the therapy outweigh the risks. When this is the case, your doctor can suggest treatment with stem cells.
Neuro-Regeneration With Mesenchymal Stem Cell Secretome
Pre-clinical research has elucidated the various effects of mesenchymal stem cells (MSCs) and their secretome on spinal cord injuries. There are several major mechanisms of injury wihich lead to functional impairment in SCI: Death of neurons and glia (support cells), interruption of axons (nerve tracts), glial scarring and loss of vasculature/blood supply.
Whilst the initial assumption, that stem cells replace lost nerve cells and thus restore function, did not prove to be true, MSCs and their secretome positively influence many of the other elements of SCI injury. They have been shown to reorganize the architecture in glial scarring and by improving the growth of axons supporting the reconnection of nerve tracts across glial scars improving the conductivity of nerve signals in the spinal cord. At the same time, MSCs have been shown to improve the vascularization and blood supply in damaged areas of the cord by inducing the growth of new blood vessels. This is a critical component in the re-establishment of a more normal micro-architecture of the spinal cord.
Give Your Body a Fighting Chance to Recover –
Get Access to the Latest Generation of Regenerative Medicine
With the latest generation of Stem Cell treatments, the Stem Cell Secretome, ANOVA, our German stem cell clinic, offers a unique and potent product that synergizes well with your physical rehabilitation recovery.
Get yourself informed of your possibilities to get treated with our stem cell-based products.
Contact our medical team at ANOVA to find out which stem cell therapy for spinal cord injury is an ideal treatment option for you.
Immunofluorescence staining of cells
Read More
Repair of injured spinal cord using biomaterial scaffolds and stem cells
Euro Stemcell foundation: How could stem cells help with spinal cord injuries
Paralysed man walks again after cell transplant
Christopher and Dana Reeve Foundation
Stem cell clinical trial aims to combat nerve damage in human tissue
Stem Cell Secretome for Spinal Cord Repair: Is It More than Just a Random Baseline Set of Factors?
!-
Frequently Asked Questions:
Stem Cell-based Treatments and Regenerative Therapies for Osteoarthritis
How Does Cortisone Work?
Injection of cortisone (corticosteroids) into inflamed joints is still widely used today. It typically results in rapid pain relief, but its effects usually do not last longer than 4 weeks. However, in the long run, cortisone injections accelerate joint wear by damaging cartilage cells, which are essential for maintaining articular cartilage.
What are NSAID, NSAP, NSAR?
All these abbreviations stand for anti-inflammatory drug groups that are used for inflammatory processes and diseases such as rheumatism, osteoarthritis and arthritis. NS always stands for non-steroidal, i.e. substances that are not derived from steroids such as cortisone. All non-steroidal anti-inflammatory drugs have sometimes serious side effects such as damage to the gastrointestinal mucosa or heart and kidney damage. Some preparations have therefore been withdrawn from the market.
- NSAID - non-steroidal anti-inflammatory drug
- NSAP - non-steroidal anti-inflammatory drug
- NSAID - non-steroidal anti-inflammatory drug (translated non-steroidal anti-inflammatory drug)
What are Anti-Phlogistic Drugs?
Anti-phlogistic drugs are anti-inflammatory drugs. Anti-inflammatory drugs include the following groups of drugs:
- Glucocorticoids (e.g., cortisone).
- Non-steroidal anti-inflammatory drugs (NSAIDs, non-steroidal anti-rheumatic drugs)
- Immunosuppressants (DMARDs, disease-modifying anti-rheumatic drugs
- Certain novel biologic-derived drugs (biologicals, e.g., JAK inhibitors)
Effects, Risks and Side Effects of Drugs and Treatments
Patients are always individuals with their own history and specific disease course. Therefore, for drugs or treatments, one can generally never guarantee an effect or grant or exclude risks and side effects. Common expectations are summarized below. However, patient-specific deviations are to be expected.
What is Bone Marrow Concentrate - BMC?
Bone Marrow Concentrate (BMC) is a source for Mesenchymal Stem Cells (MSCs) and Hematopoietic Stem cells (HSC). It contains many important growth and regenerative factors, in addition to the MSC and HSC in natural composition. The BMC procedure is relatively simple and minimally invasive, therefore it has been a favorite source for stem cell-based therapies in the previous decades. Many clinics rely on BMC as their main stem cell treatment, sometimes with exaggerated claims. However, BMC has demonstrated impressive results for effective treatment of numerous diseases, among them being osteoarthritis. Read more about our BMC Treatment here.
What is Platelet Rich Plasma - PRP?
Platelet Rich Plasma (PRP) is a blood-derived, cellular product with concentrated supply of regenerative growth factors and cytokines, obtained from the patient's own blood. It is very simple to acquire, and it has shown promising results in the treatment of several inflammatory and degenerative diseases. For the treatment of specific diseases, it can be combined with BMC or adMSCs, as it has synergistic additive effects to the treatment. PRP has "special" functions: it serves as a growth medium to maintain stem cells healthy; ensures adequate cellular environment where enough energy is provided to allow the cells to perform their regenerative work.
Is Therapeutic Success Guaranteed?
No therapy can guarantee a 100% success after treatment. However, in the case of experimental therapies such as stem cell therapy, the attending physician must perform a benefit-to-risk analysis for each patient and determine both the benefits and the risks for that particular patient. If the potential benefit outweighs the potential side effects, the doctor may recommend experimental therapy.
-->
References and Literature - Stem Cell-based Therapies and Spinal Cord Injury
- A. Hejcl, J. Sedy, M. Kapcalova, D.A. Toro, T. Amemori, P. Lesny, K. Likavcanova-Masinova, E. Krumbholcova, M. Pradny, J. Michalek, M. Burian, M. Hajek, P. Jendelova, E. Sykova, HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury, Stem Cells Dev. 19 (2010) 1535e1546.
- Anthony, Diana F., and Paul G. Shiels. "Exploiting paracrine mechanisms of tissue regeneration to repair damaged organs." Transplantation research 2.1 (2013): 10.
- Wright KT, Masri WE, Osman A, Chowdhury J, Johnson WEB: Concise review: bone marrow for the tre atment of spinal cord injury: mechanisms and clinical implications. Stem Cells 2011, 29: 169 – 178
- Quertainmont R, Cantinieaux D, Bot man O, Eid S, Schoenen J, Franzen R: Mesenchymalstemcellgraftimproves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One 2012, 7: e39500.
- Lamichhane, Tek N., et al. "Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine." Tissue Engineering Part B: Reviews 21.1 (2014): 45-54.
- Thuret, Sandrine, Lawrence DF Moon, and Fred H. Gage. "Therapeutic interventions after spinal cord injury." Nature Reviews Neuroscience 7.8 (2006): 628-643.
- Thuret, Sandrine, Lawrence DF Moon, and Fred H. Gage. "Therapeutic interventions after spinal cord injury." Nature Reviews Neuroscience 7.8 (2006): 628-643.
- Lindvall O, Kokaia Z: Stem cells for the treatment of neurological disorders. Nature 2006, 441(7097):1094-1096.
- Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, et al: Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells 2007, 25(8):2066-2073.
- Karamouzian S, Nematollahi-Mahani SN, Nakhaee N et al (2012) Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg 114(7):935–939
- Saito F, Nakatani T, Iwase M et al (2008) Spinal cord injury treatment with intrathecal autologous bone marrow stromal cell transplantation: the first clinical trial case report. J Trauma 64(1):53–59
- Saito F, Nakatani T, Iwase M et al (2012) Administration of cul¬tured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor Neurol Neu¬rosci 30(2):127–136
Further References about MSEC and Stemcell Therapies for Spinal Cord Injuries
- Ramer L.M., Ramer M.S., Bradbury E.J. Restoring function after spinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol. 2014;13:1241–1256. https://doi.org/10.1016/S1474-4422(14)70144-9.
- Silva N.A., Sousa N., Reis R.L., Salgado A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014;114:25–57. https://doi.org/10.1016/j.pneurobio.2013.11.002.
- Bradbury E.J., Burnside E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019;10:3879. https://doi.org/10.1038/s41467-019-11707-7.
- Schuld C., Franz S., Bruggemann K., Heutehaus L., Weidner N., Kirshblum S.C., Rupp R., EMSCI study group International standards for neurological classification of spinal cord injury: Impact of the revised worksheet (revision 02/13) on classification performance. J. Spinal Cord Med. 2016;39:504–512. https://doi.org/10.1080/10790268.2016.1180831.
- Anderson D.K., Means E.D., Waters T.R., Green E.S. Microvascular perfusion and metabolism in injured spinal cord after methylprednisolone treatment. J. Neurosurg. 1982;56:106–113. https://doi.org/10.3171/jns.1982.56.1.0106.
- Oyinbo C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011;71:281–299.
- Kakulas B.A. Neuropathology: The foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–563. https://doi.org/10.1038/sj.sc.3101670.
- Rowland J.W., Hawryluk G.W., Kwon B., Fehlings M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus. 2008;25:E2. https://doi.org/10.3171/FOC.2008.25.11.E2
- Donnelly D.J., Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. https://doi.org/10.1016/j.expneurol.2007.06.009
- Pires A.O., Mendes-Pinheiro B., Teixeira F.G., Anjo S.I., Ribeiro-Samy S., Gomes E.D., Serra S.C., Silva N.A., Manadas B., Sousa N., et al. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue-Derived Stem Cells, and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016;25:1073–1083. https://doi.org/10.1089/scd.2016.0048.
- Nagoshi N., Nakashima H., Fehlings M.G. Riluzole as a neuroprotective drug for spinal cord injury: From bench to bedside. Molecules. 2015;20:7775–7789. https://doi.org/10.3390/molecules20057775.
- Salewski R.P., Mitchell R.A., Li L., Shen C., Milekovskaia M., Nagy A., Fehlings M.G. Transplantation of Induced Pluripotent Stem Cell-Derived Neural Stem Cells Mediate Functional Recovery Following Thoracic Spinal Cord Injury Through Remyelination of Axons. Stem Cells Transl. Med. 2015;4:743–754. https://doi.org/10.5966/sctm.2014-0236
- Zakrzewski W., Dobrzynski M., Szymonowicz M., Rybak Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019;10:68. https://doi.org/10.1186/s13287-019-1165-5.
- Chen C.J., Ou Y.C., Liao S.L., Chen W.Y., Chen S.Y., Wu C.W., Wang C.C., Wang W.Y., Huang Y.S., Hsu S.H. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp. Neurol. 2007;204:443–453. https://doi.org/10.1016/j.expneurol.2006.12.004
Literature on progressive neuromuscular diseases and HAL training
- Grasmücke D., Zieriacks A., Jansen O., Fisahn Ch., Sczesny-Kaiser M., Wessling M., Meindl R.C., Schildhauer T.A., Aach M. Against the odds: what to expect in rehabilitation of chronic spinal cord injury with a neurologically controlled Hybrid Assistive Limb exoskeleton. A subgroup analysis of 55 patients according to age and lesion level. Journal of Neurosurgery Vol. 42: Issue 5 https://doi.org/10.3171/2017.2.FOCUS171
- Brinkemper A., Grasmücke D., Yilmaz E., Reinecke F., Schildhauer T. A., Aach M. Influence of Locomotion Therapy With the Wearable Cyborg HAL on Bladder and Bowel Function in Acute and Chronic SCI Patients. Global Spine Journal April 16, 2021 https://doi.org/10.1177/21925682211003851
- Jansen O., Schildhauer T. A., Meindl R. C., Tegenthoff M., Schwenkreis T., Sczesny-Kaiser M., Grasmücke D., Fisahn Ch. Aach M. Functional Outcome of Neurologic-Controlled HAL-Exoskeletal Neurorehabilitation in Chronic Spinal Cord Injury: A Pilot With One Year Treatment and Variable Treatment Frequency.Global Spine Journal July 7, 2017 https://doi.org/10.1177/2192568217713754
- Jansen O., Grasmücke D., Meindl R. C., Tegenthoff M., Schwenkreis P., Sczesny-Kaiser M., Wessling M. Schildhauer T.A., Fisahn Ch., Aach M. Hybrid Assistive Limb Exoskeleton HAL in the Rehabilitation of Chronic Spinal Cord Injury: Proof of Concept; the Results in 21 Patients. World Neurosurgery, February2, 2018 https://doi.org/10.1016/j.wneu.2017.10.080
- Sczesny-Kaiser M., Höffken O., Aach M., Cruciger O., Grasmücke D., Meindl R., Schildhauer T.A., Schwenkreis P., Tegenthoff M. HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. Journal of NeuroEngineering and Rehabilitation, August 20, 2015 https://doi.org/10.1186/s12984-015-0058-9
- Aach M., Cruciger O., Sczesny-Kaiser M., Höffken O., Meindl R. C., Tegenthoff M., Schwenkreis P., Sankai Y., Schildhauer T. A. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: a pilot study. The Spine Journal April 07, 2014 https://doi.org/10.1016/j.spinee.2014.03.042
- Puentes S., Kadone H., Kubota S., Abe T., Shimizu Y., Marushima A., Sankai Y., Yamazaki M., Suzuki K. Reshaping of Gait Coordination by Robotic Intervention in Myelopathy Patients After Surgery. Frontiers in Neuroscience March 02, 2018 https://doi.org/10.3389/fnins.2018.00099
- Cruciger O., Schildhauer T. A., Meindl R. C., Twegenthoff M., Schwenkreis P., Citak M., Aach M. Impact of locomotion training with a neurologic controlled hybrid assistive limb (HAL) exoskeleton on neuropathic pain and health related quality of life (HRQoL) in chronic SCI: a case study. Disability and Rehabilitation: Assistive Technology November 10, 2014 https://doi.org/10.3109/17483107.2014.981875
Further References for MSC, BMC, Stemcell Secretome and EVs
- Georg Hansmann, Philippe Chouvarine, Franziska Diekmann, Martin Giera, Markus Ralser, Michael Mülleder, Constantin von Kaisenberg, Harald Bertram, Ekaterina Legchenko & Ralf Hass "Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension". Nature Cardiovascular Research volume 1, pages568–576 (2022).
- Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis . Arthritis Rheum. 2003;48:3464–74.
- Lee KB, Hui JH, Song IC, Ardany L, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cell. 2007;25:2964–71.
- Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy . 2009;25(12):1391–400.
- Black L, Gaynor J, Adams C, et al. Effect of intra-articular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200.
- Centeno C, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–53.
- Centeno C, Kisiday J, Freeman M, et al. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: a case study. Pain Physician. 2006;9:253–6.
- Centeno C, Schultz J, Cheever M. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell. 2011;5(1):81–93.
- Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose derived stem cells: a case series. J Med Case Rep. 2001;5:296.
- Kuroda R, Ishida K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31.
- Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422–8.
- Saw KY et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94.
- Vangsness CT, Farr J, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg. 2014;96(2):90–8.
- Freitag, Julien, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy–a review. BMC musculoskeletal disorders 17.1 (2016): 230.
- Maumus, Marie, Christian Jorgensen, and Danièle Noël. " Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. " Biochimie 95.12 (2013): 2229-2234.
- Dostert, Gabriel, et al. " How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication?. " Frontiers in Cell and Developmental Biology 5 (2017).
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- Toh, Wei Seong, et al. " MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. " Seminars in Cell & Developmental Biology. Academic Press, 2016.
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- S. Koelling, J. Kruegel, M. Irmer, J.R. Path, B. Sadowski, X. Miro, et al., Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis , Cell Stem Cell 4 (2009) 324–335.
- B.A. Jones, M. Pei, Synovium-Derived stem cells: a tissue-Specific stem cell for cartilage engineering and regeneration , Tissue Eng. B: Rev. 18 (2012) 301–311.
- W. Ando, J.J. Kutcher, R. Krawetz, A. Sen, N. Nakamura, C.B. Frank, et al., Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage, Cytotherapy 16 (2014) 776–788.
- K.B.L. Lee, J.H.P. Hui, I.C. Song, L. Ardany, E.H. Lee, Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model, Stem Cells 25 (2007) 2964–2971.
- W.-L. Fu, C.-Y. Zhou, J.-K. Yu, A new source of mesenchymal stem cells for articular cartilage repair: mSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model , Am. J. Sports Med. 42 (2014) 592–601.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, et al., Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration , Biomaterials 33 (2012) 7008–7018.
- E.-R. Chiang, H.-L. Ma, J.-P. Wang, C.-L. Liu, T.-H. Chen, S.-C. Hung, Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits , PLoS One 11 (2016) e0149835.
- H. Nejadnik, J.H. Hui, E.P. Feng Choong, B.-C. Tai, E.H. Lee, Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study , Am. J. Sports Med. 38 (2010) 1110–1116.
- I. Sekiya, T. Muneta, M. Horie, H. Koga, Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects , Clin. Orthop. Rel. Res. 473 (2015) 2316–2326.
- Y.S. Kim, Y.J. Choi, Y.G. Koh, Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes , Am. J. Sports Med. 43 (2015) 2293–2301.
- W.-L. Fu, Y.-F. Ao, X.-Y. Ke, Z.-Z. Zheng, X. Gong, D. Jiang, et al., Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment , Knee 21 (2014) 609–612.
- Y.-G. Koh, O.-R. Kwon, Y.-S. Kim, Y.-J. Choi, D.-H. Tak, Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial , Arthrosc. J. Arthrosc. Relat. Surg. 32 (2016) 97–109.
- T.S. de Windt, L.A. Vonk, I.C.M. Slaper-Cortenbach, M.P.H. van den Broek, R. Nizak, M.H.P. van Rijen, et al., Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-Stage cartilage repair in humans upon mixture with recycled autologous chondrons , Stem Cells (2016) (n/a-n/a).
- L. da Silva Meirelles, A.M. Fontes, D.T. Covas, A.I. Caplan, Mechanisms involved in the therapeutic properties of mesenchymal stem cells , Cytokine Growth Factor Rev. 20 (2009) 419–427.
- W.S. Toh, C.B. Foldager, M. Pei, J.H.P. Hui, Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration , Stem Cell Rev. Rep. 10 (2014) 686–696.
- R.C. Lai, F. Arslan, M.M. Lee, N.S.K. Sze, A. Choo, T.S. Chen, et al., Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury , Stem Cell Res. 4 (2010) 214–222.
- S. Zhang, W.C. Chu, R.C. Lai, S.K. Lim, J.H.P. Hui, W.S. Toh, Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration, Osteoarthr . Cartil. 24 (2016) 2135–2140.
- S. Zhang, W. Chu, R. Lai, J. Hui, E. Lee, S. Lim, et al., 21 – human mesenchymal stem cell-derived exosomes promote orderly cartilage regeneration in an immunocompetent rat osteochondral defect model , Cytotherapy 18 (2016) S13.
- C.T. Lim, X. Ren, M.H. Afizah, S. Tarigan-Panjaitan, Z. Yang, Y. Wu, et al., Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model
- A. Gobbi, G. Karnatzikos, S.R. Sankineani, One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee , Am. J. Sports Med. 42 (2014) 648–657.
- A. Gobbi, C. Scotti, G. Karnatzikos, A. Mudhigere, M. Castro, G.M. Peretti, One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years , Knee Surg. Sports Traumatol. Arthrosc. (2016) 1–8.
- A. Gobbi, G. Karnatzikos, C. Scotti, V. Mahajan, L. Mazzucco, B. Grigolo, One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-Year follow-up , Cartilage 2 (2011) 286–299.
- K.L. Wong, K.B.L. Lee, B.C. Tai, P. Law, E.H. Lee, J.H.P. Hui, Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up , Arthrosc. J. Arthrosc. Relat. Surg. 29 (2013) 2020–2028.
- J.M. Hare, J.E. Fishman, G. Gerstenblith, et al., Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the poseidon randomized trial, JAMA 308 (2012) 2369–2379.
- L. Wu, J.C.H. Leijten, N. Georgi, J.N. Post, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation , Tissue Eng. A 17 (2011) 1425–1436.
- L. Wu, H.-J. Prins, M.N. Helder, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells in chondrocyte Co-Cultures are independent of culture conditions and cell sources , Tissue Eng. A 18 (2012) 1542–1551.
- S.K. Sze, D.P.V. de Kleijn, R.C. Lai, E. Khia Way Tan, H. Zhao, K.S. Yeo, et al., Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells , Mol. Cell. Proteomics 6 (2007) 1680–1689.
- M.B. Murphy, K. Moncivais, A.I. Caplan, Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine , Exp. Mol. Med. 45 (2013) e54.
- M.J. Lee, J. Kim, M.Y. Kim, Y.-S. Bae, S.H. Ryu, T.G. Lee, et al., Proteomic analysis of tumor necrosis factor--induced secretome of human adipose tissue-derived mesenchymal stem cells , J. Proteome Res. 9 (2010) 1754–1762.
- S. Bruno, C. Grange, M.C. Deregibus, R.A. Calogero, S. Saviozzi, F. Collino, et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury, J. Am. Soc. Nephrol. 20 (2009) 1053–1067.
- M. Yá˜nez-Mó, P.R.-M. Siljander, Z. Andreu, A.B. Zavec, F.E. Borràs, E.I. Buzas, et al. Biological properties of extracellular vesicles and their physiological functions (2015).
- C. Lawson, J.M. Vicencio, D.M. Yellon, S.M. Davidson, Microvesicles and exosomes: new players in metabolic and cardiovascular disease , J. Endocrinol. 228 (2016) R57–R71.
- A.G. Thompson, E. Gray, S.M. Heman-Ackah, I. Mager, K. Talbot, S.E. Andaloussi, et al., Extracellular vesicles in neurodegenerative diseas—pathogenesis to biomarkers, Nat. Rev. Neurol. 12 (2016) 346–357.
- I.E.M. Bank, L. Timmers, C.M. Gijsberts, Y.-N. Zhang, A. Mosterd, J.-W. Wang, et al., The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease , Expert Rev. Mol. Diagn. 15 (2015) 1577–1588.
- T. Kato, S. Miyaki, H. Ishitobi, Y. Nakamura, T. Nakasa, M.K. Lotz, et al., Exosomes from IL-1 stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes , Arthritis. Res. Ther. 16 (2014) 1–11.
- R.W.Y. Yeo, S.K. Lim, Exosomes and their therapeutic applications, in: C. Gunther, A. Hauser, R. Huss (Eds.), Advances in Pharmaceutical Cell TherapyPrinciples of Cell-Based Biopharmaceuticals, World Scientific, Singapore, 2015, pp. 477–491.
- X. Qi, J. Zhang, H. Yuan, Z. Xu, Q. Li, X. Niu, et al., Exosomes secreted by human-Induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats , Int. J. Biol. Sci. 12 (2016) 836–849.
- R.C. Lai, F. Arslan, S.S. Tan, B. Tan, A. Choo, M.M. Lee, et al., Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles , J. Mol. Cell. Cardiol. 48 (2010) 1215–1224.
- Y. Zhou, H. Xu, W. Xu, B. Wang, H. Wu, Y. Tao, et al., Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro , Stem Cell Res. Ther. 4 (2013) 1–13.
- Y. Qin, L. Wang, Z. Gao, G. Chen, C. Zhang, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo , Sci. Rep. 6 (2016) 21961.
- M. Nakano, K. Nagaishi, N. Konari, Y. Saito, T. Chikenji, Y. Mizue, et al., Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes , Sci. Rep. 6 (2016) 24805.
- K. Nagaishi, Y. Mizue, T. Chikenji, M. Otani, M. Nakano, N. Konari, et al., Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes , Sci. Rep. 6 (2016) 34842.
- S.R. Baglio, K. Rooijers, D. Koppers-Lalic, F.J. Verweij, M. Pérez Lanzón, N. Zini, et al., Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species , Stem Cell Res. Ther. 6 (2015) 1–20.
- T. Chen, R. Yeo, F. Arslan, Y. Yin, S. Tan, Efficiency of exosome production correlates inversely with the developmental maturity of MSC donor, J. Stem Cell Res. Ther. 3 (2013) 2.
- R.C. Lai, S.S. Tan, B.J. Teh, S.K. Sze, F. Arslan, D.P. de Kleijn, et al., Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome , Int. J. Proteomics 2012 (2012) 971907.
- T.S. Chen, R.C. Lai, M.M. Lee, A.B.H. Choo, C.N. Lee, S.K. Lim, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs , Nucleic Acids Res. 38 (2010) 215–224.
- R.W. Yeo, R.C. Lai, K.H. Tan, S.K. Lim, Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell, J. Circ. Biomark. 1 (2013) 7.
- R.C. Lai, R.W. Yeo, S.K. Lim, Mesenchymal stem cell exosomes, Semin. Cell Dev. Biol. 40 (2015) 82–88.
- B. Zhang, R.W. Yeo, K.H. Tan, S.K. Lim, Focus on extracellular vesicles: therapeutic potential of stem cell-derived extracellular vesicles , Int. J. Mol. Sci. 17 (2016) 174.
- Hu G-w, Q. Li, X. Niu, B. Hu, J. Liu, Zhou S-m, et al., Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice , Stem Cell Res. Ther. 6 (2015) 1–15.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li, et al., Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis , J. Transl. Med. 13 (2015) 1–14.
- B. Zhang, M. Wang, A. Gong, X. Zhang, X. Wu, Y. Zhu, et al., HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing, Stem Cells 33 (2015) 2158–2168.
- B. Zhang, Y. Yin, R.C. Lai, S.S. Tan, A.B.H. Choo, S.K. Lim, Mesenchymal stem cells secrete immunologically active exosomes , Stem Cells Dev. 23 (2013) 1233–1244.
- C.Y. Tan, R.C. Lai, W. Wong, Y.Y. Dan, S.-K. Lim, H.K. Ho, Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models , Stem Cell Res. Ther. 5 (2014) 1–14.
- C. Lee, S.A. Mitsialis, M. Aslam, S.H. Vitali, E. Vergadi, G. Konstantinou, et al., Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension , Circulation 126 (2012) 2601–2611.
- B. Yu, H. Shao, C. Su, Y. Jiang, X. Chen, L. Bai, et al., Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1 , Sci. Rep. 6 (2016) 34562.
- Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof of concept clinical trial. Stem Cells. 2014;32(5):1254–66.
- Vega, Aurelio, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
- Davatchi F, Sadeghi-Abdollahi B, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–5
- Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case- controlled study. Int Orthop. 2014;38(9):1811–1818
- Galli D, Vitale M, Vaccarezza M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:6.
- Beitzel K, Solovyova O, Cote MP, et al. The future role of mesenchymal Stem cells in The management of shoulder disorders . Arthroscopy. 2013;29(10):1702–1711.
- Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190.
- Malda, Jos, et al. " Extracellular vesicles [mdash] new tool for joint repair and regeneration. " Nature Reviews Rheumatology (2016).
Further References about PRP
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4(1):52–62.
Extras
- Xu, Ming, et al. " Transplanted senescent cells induce an osteoarthritis-like condition in mice. " The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (2016): glw154.
- McCulloch, Kendal, Gary J. Litherland, and Taranjit Singh Rai. " Cellular senescence in osteoarthritis pathology ." Aging Cell (2017).
Patient Services at ANOVA Institute for Regenerative Medicine
- Located in the center of Germany, quick access by car or train from anywhere in Europe
- Simple access worldwide, less than 20 minutes from Frankfurt Airport
- Individualized therapy with state-of-the-art stem cell products
- Individually planned diagnostic work-up which include world-class MRI and CT scans
- German high quality standard on safety and quality assurance
- Personal service with friendly, dedicated Patient Care Managers
- Scientific collaborations with academic institutions to assure you the latest regenerative medical programs