Stem Cell Treatment Program for Erectile Dysfunction
at ANOVA IRM in Offenbach, Germany
Erectile Dysfunction (ED) is a problem which affects approx. 50% of all men between the ages of 40 to 70 years . ED is defined as the inability to have an erection of the penis that is sufficient for sexual intercourse .
In about 80% of cases, a physical cause can be identified , including cardiovascular disease, diabetes mellitus, drug side effects and neurological problems that may occur after surgical removal of the prostate or similar procedures. Physicians speak of psychological impotence when an erection or penetration is impossible or impaired due to thoughts or feelings; this problem occurs in approx. 10% of all ED cases.
On this page we inform you about erectile dysfunction covering an overview on important aspects of causes, treatment options, precision diagnostics as well as our stem cell-based therapies that we offer in Offenbach (near Frankfurt am Main, FRA Airport) Germany.
Jump directly to the following topics:
- Conventional therapies
- ANOVA therapies for ED
- Expectations and limits
- Our ED treatment options
- Workflow of the treatment process
- Precision diagnostics
- The ANOVA difference: targeted treatment
- You want a second opinion
- FAQ- frequently-asked questions
- Sources and Literature
ED Conventional Treatments - Medication - Diagnostics
Whilst many men afflicted with ED nowadays, profit from treatment with PDE5I (phosphodiesterase type-5 inhibitors)-based medication such as Viagra or Cialis , these drugs can cause unpleasant side effects, are contraindicated in some patients and do not always provide the desired results.
Other treatments for ED include injections into the penis or the urethra, vacuum erection devices, and penile prosthesis implantation. These options are considered undesirable by most men because of their invasiveness, artificiality and unsatisfactory outcomes .
All of these methods solely treat the symptoms of ED but do not cure the underlying disease process. This might now change with the availability of stem cell-based therapies.
Physiology of Erection and Causes of Erectile Dysfunction
Erection of the penis is achieved by filling of the erectile bodies of the penis with blood. Triggered by psychological stimuli, nerve signals from the brain are sent via the spinal cord to the peripheral nerves, which then release nitric oxide (NO, a signalling molecule) in the erectile bodies. NO causes the smooth muscles of the erectile bodies to relax via the release of cGMP (cyclic guanosine monophosphate, a so-called second messenger, secondary signalling molecule inside the cells), and to engorge with blood. This is the trigger phase of an erection which requires the nerves to function normally.
The enlargement of the erectile bodies in turn compresses the draining veins, between the erectile bodies and the tunica albuginea of the penis, resulting in occlusion of venous outflow and full erection . This is the mechanical phase of an erection.
Detumescence (flaccidity) of the penis occurs when the second messenger cGMP is degraded by an enzyme, called type 5 phosphodiesterase, leading to contraction of the smooth muscles of the erectile bodies, which results in decreased inflow of blood, a consequent reduction in the size of the erectile bodies and a de-compression of the veins, effecting the drainage of blood from the erectile bodies. The key components of an erection are the endothelial cells (EC) and the smooth muscle cells of the erectile bodies, the corpora cavernosa (CSMC), and the release of NO from the cavernosal nerves (CN).
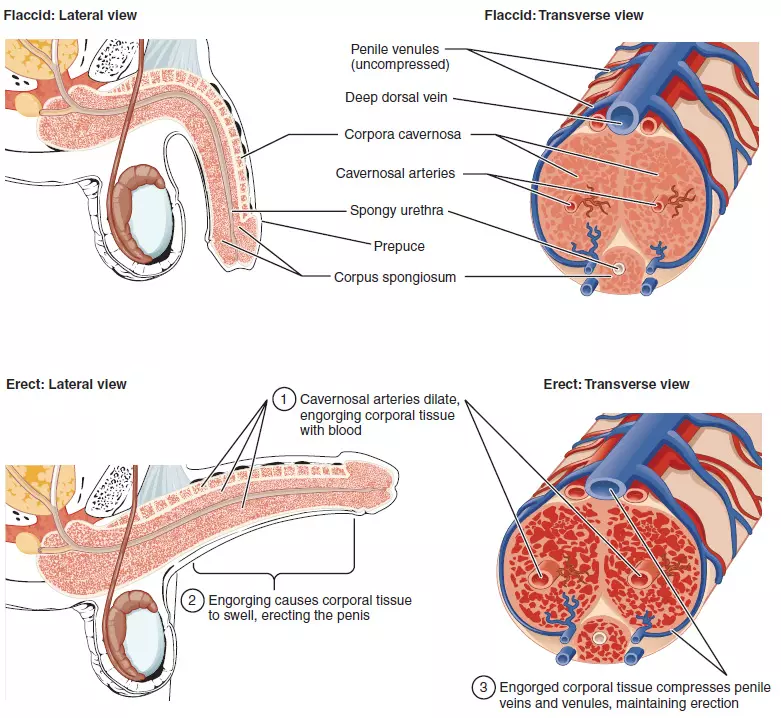
The erectile system of the penis “Cross-Sectional Anatomy of the Penis” by Philschatz. (https://philschatz.com/anatomy-book/contents/m46400.html) License: CC BY 4.0, ANOVA IRM - Germany
Aging and a variety of diseases can alter these essential anatomical and physiological components, resulting in ED. After radical prostatectomy (RPE, complete removal of the prostate), the standard treatment for prostate cancer, the cavernosal nerves can be damaged . Whilst the immediately resulting neurogenic ED might be reversible, the long-term consequences, such as diminished NO production, and death of the smooth muscle cells of the erectile bodies can lead to atrophy and fibrosis of the erectile bodies, might result in permanent ED. High blood glucose levels in diabetes mellitus , and increased blood cholesterol and hyperlipidemia can also cause ED by similar mechanisms.
ED Diagnostics at ANOVA IRM - Reveal the Cause and Stage
As there are many causes of ED, a comprehensive diagnostic work-up is needed to find the problem and in turn the right treatment. At ANOVA, we perform the following tests:
- ED questionnaire to assess general health and specific problems
- Physical exam by Urologist/Andrologist
- Comprehensive blood tests including hormone values
(testosterone, etc.) - Ultrasound imaging of the penis and penile arteries
(doppler and duplex US) - Magnetic resonance imaging (MRI) and angiography (MRA) of the arteries supplying the penis
Read more about diagnostics for ED below.
Stem Cell Treatments for Erectile Dysfunction at
ANOVA Institute for Regenerative Medicine - Offenbach, Germany
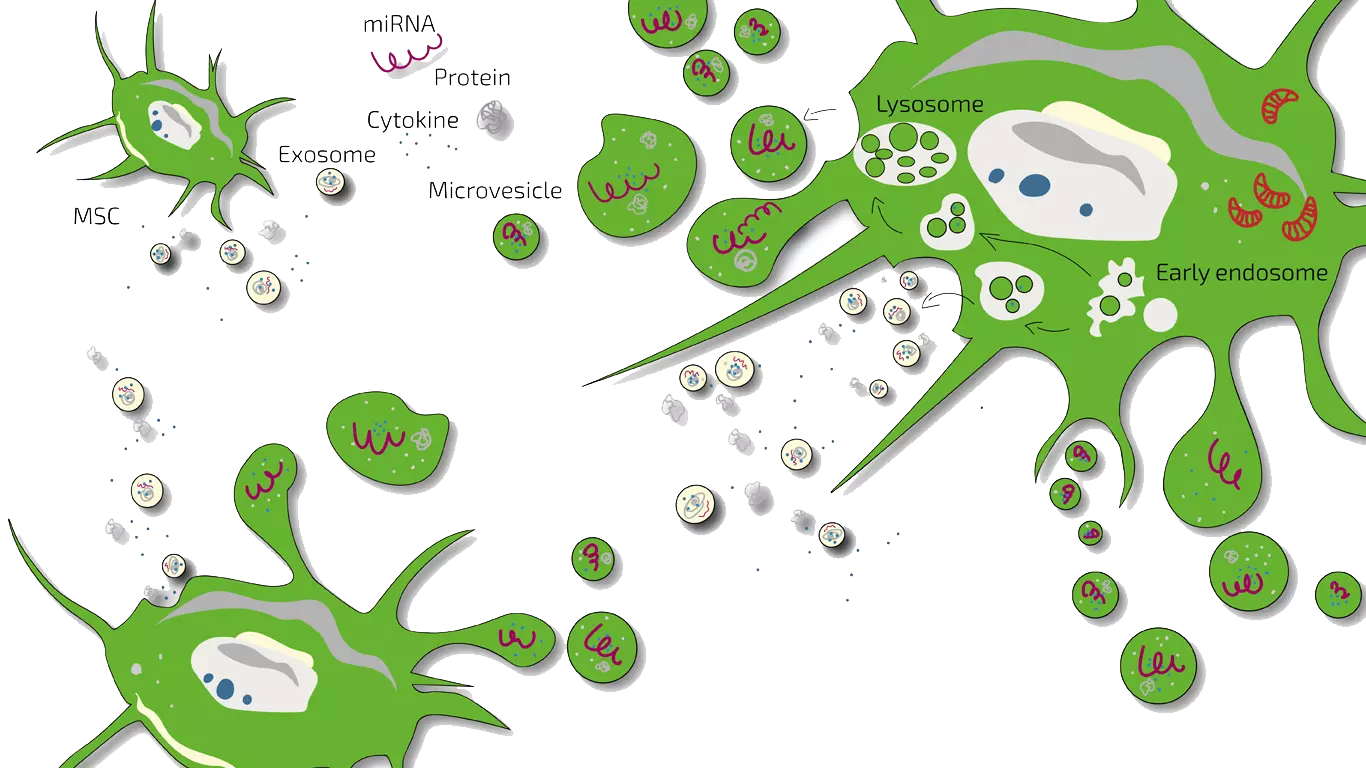
Secretome/Exosomes
Potency Hypothesis of Stem Cell Therapies
Stem cells possess the potential to communicate with the immune cells that elicit the inflammation and by natural, so far not understood mechanisms may inhibit an immune-over-reaction. Furthermore, stem cells have the ability to stimulate regeneration of tissue thereby counteracting the loss of functionality.
Over the past few years, particular enthusiasm has developed for stem cell-based therapies in the urological field, especially for the treatment of erectile dysfunction. Several pre-clinical studies have researched the application of stem cells for urological conditions, particularly bone marrow stem cells (BMSC) and adipose-derived stem cells (ADSCs) for the treatment of ED in animal models, summarized by Soebadi et al. in 2016.
In cases of acute ED, the mechanism of repair effected by stem cells is assumed to be on a paracrine level. In chronic ED, however, the effect of stem cells may be mainly based on engraftment and cellular differentiation. The exact mechanism of how stem cells improve erectile function in chronic ED is still not fully solved.
MSEC - Mesenchymal Stem Cell Secretome - Exosomes - Autologous
Patients with a prolonged history of ED symptoms are usually treated with MSEC (secretome, exosomen, EVs) of mesenchymal stem cells (MSC, AD-MSC, adipose-derived, fat-derived stem cells) which we harvest from the patients belly in a mini-liposuction (very brief and limited liposuction) under slight sedation. Worldwide, ANOVA is the first stem cell clinic to acquire legal permission form the responsible governmental authorities and therefore, offers high quality, safe and legally-controlled autologous (own) exosome-containing secretome.
The main advantage of MSEC is that in contrast to live stem cells which would loose their therapeutic potency, can be frozen without loss of exosomes. This enables us to produce 10-20 injection doses from one liposuction which can then be administered over a longer treatment period. This is especially advantageous for long-term stimulation of regeneration in ED.
What a Secretome/Exosome is and how they compare is explained on our overview page.
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
Therapy Workflow for Erectile Dysfunction
The precise workflow is described in detail on the stem cell- specific pages of BMC (most often used for OA), Secretome/Exosomes and PRP (as combination therapy).
All therapies are divided into phases such as evaluation of the medical history (we analyze your current therapies and medical records), initial counseling and evaluation of potential, patient-individual benefit of a stem cell therapy (indication statement), preliminary examinations, diagnostics, consultation on all therapy options, preparation of an individual treatment plan including cost estimate, harvesting of tissue, production of the stem cell product, quality control of the product and application. There are two special features for osteoarthritis and arthritis patients. If your previous findings have not found the specific causes of your joint pain, we will examine you in advance with a precise and informative arthro-MRI or an MRI with non-radioactive contrast medium, if you wish. In addition, we often apply the stem cells (BMC) intra-articularly (i.e., directly in the joint). This means that we deliver the stem cells to the exact location where your pain originates.
Unfortunately, according to the risk-benefit ratio, we cannot treat children or pregnant women. In addition, other factors can also be exclusion criteria.
How Long Does a Stem Cell Therapy Take?
The initial analyses and counseling can be done without you having to travel to Offenbach (near Frankfurt/Main, Germany). This period can be 2 weeks up to months depending on the availability of patients slots. If you live further away, we will conduct the initial discussions by telephone or video conference. For the actual treatment, you will travel to Offenbach. Then, depending on the therapy, the tissue collection, quality control and treatment type it will take as follows:
BMC- and PRP-Therapy
Each donation and application of BMC on-site period: 2 days (consecutive days).
Secretome/exosome-Therapy:
Preparation and harvest of the fat (mini-liposuction) need once 2 days (consecutive days) in Offenbach, followed by enrichment of the mesenchymal stem cells (secretome/exosome) and quality control. Approximately 4 weeks after the isolation, the therapy begins according to the therapy plan determined with you. You will then come to Offenbach am Main (Germany) several times for the application. The shelf life of the secretome (exosomes) is 2 years.
How Much Does Stem Cell Treatment Cost?
Our treatments are always tailored to your specific situation, disease, stage and other factors. The therapies differ in the product used (BMC, secretome, PRP or hyaluronic acid), the frequency of treatment as well as the further examinations and your sedation and anesthesia wishes. A treatment for erectile dysfunction can cost from a few thousand to several thousand euros. You will receive a cost estimate for all treatments in advance so that you can accurately estimate what a treatment would cost in your individual case.
Does my Health Insurance Cover the Therapy Costs?
Unfortunately, at the moment it is assumed that health insurance companies do not cover the costs of experimental therapies (BMC, secretome, PRP), i.e. you will have to bear the costs entirely yourself.
How Does the ANOVA Therapy Differ?
Diagnostics – We Look for the Cause of your Problem
Dr. mult. Michael K. Stehling, the founder of ANOVA IRM and the Vitus Prostate Center , is a radiologist (MD) and holds a PhD in physics. For this reason, the ANOVA Institute for Regenerative Medicine, in cooperation with the Prof. Stehling Institute for Diagnostic Imaging located in the same building, has the capability to use special precision diagnostics such as ultrasound imaging of the penis and penile arteries (doppler and duplex US) as well as magnetic resonance imaging (MRI) and angiography (MRA) of the arteries supplying the penis.
Compared to many conventional MRIs, these methods are often able to localize the cause of the condition. This enables us to determine individually how patients should be treated and where the stem cells should be applied.
Furthermore, in consultation with you, we supplement our patient-specific diagnostics with specific blood tests on hormones, inflammation parameters and other factors that are important in your case, or recommend further examinations such as a preventive MRI scan.
Diagnostic Considerations:
For the individual, patient-specific and successful treatment of ED a thorough diagnostic-work-up is necessary to identify the cause and the stage of ED and to rule out other conditions as the initiating factors.
Conditions to be Taken Into Consideration:
- Cancer and cancer treatment
- Hypogonadism
- Performance anxiety
- Medications:
- Antidepressants
- Antipsychotics
- Antihypertensives
- Antiulcer drugs
- Hyperlipidemia medications
Differential Diagnoses:
- Abdominal Vascular Injuries
- Depression
- Diabetes Mellitus Type 2
- Hemochromatosis
- Hypertension
- Hypogonadism
- Hypopituitarism (Panhypopituitarism)
- Noncoronary Atherosclerosis
Laboratory Investigation for Erectile Dysfunction - ED:
- Hemoglobin A1c
- Serum chemistry panel
- Lipid profile
- Urinalysis
- Red blood cells (RBCs)
- White blood cells (WBCs)
- Protein
- Glucose
Evaluation of Hormonal Status:
- Morning serum testosterone levels (testosterone levels peak at approximately 8 am)
- Measuring total, free, and bioavailable testosterone levels and serum Hormone–binding globulin
- Luteinizing hormone (LH)
- Follicle stimulating hormone (FSH)
- Prolactin
- Thyroid-stimulating hormone (TSH)
How Does the ANOVA Therapy Differ?
We Implant the Stem Cells Precisely Where They are Needed
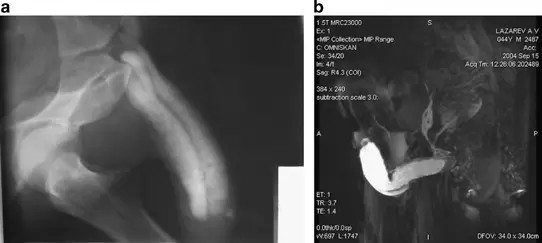
Erectile dysfunction - diagnostics:
A 43-year-old patient: (a) pharmacocavernoso-graphy does not show venous leakage clearly. (b) MRI: significant venous leakage (mixed type) is visualized including caverno-balanic shunt. ANOVA IRM - Germany
Based on our specific diagnostics using ultrasound imaging of the penis and penile arteries (doppler and duplex US) as well as magnetic resonance imaging (MRI) and angiography (MRA) of the arteries supplying the penis, we can, in contrast to many other clinics, deliver the stem cells with image support, e.g. using CT, precisely to the affected area. This means we can inject into and at the site of damage or cause to specifically and quickly trigger an effect where needed. All interventions are performed under supervision and care of our anesthesiologist and are pain free.
A purely intravenous administration, as many other clinics do, is only performed for the secretome (exosomes) if this is to be used additionally as a supportive or preventive measure because joint problems are present in several places in the body as the secretome is aimed to centrally modulate the immune response in order to inhibit over-reactions.
Of course, we will thoroughly advise you in the early process and the on-site consultation in advance on all steps and discuss alternatives and expectations.
Safe Methods of Stem Cell Delivery in Erectile Dysfunction
There are different possible methods of application. The intravenous injection of ADSCs has been shown to improve erectile function . Injection of stem cells into the erectile bodies of the penis (intra-corporal) has also been applied, since it is both easy and appears logical . Peri-prostatic injection, with or without a concurrent intra-corporal injection, has also been performed , . All of these application forms can potentially help, as the regenerative effect of stem cells is mainly achieved by either indirectly by secreting growth factors locally via paracrine mechanisms or by direct migration of the cells to the major pelvic ganglia .
The safety, efficacy, and the mechanisms of both bone marrow (BM) and adipose tissue derived (ADSC) stem-cells for the treatment of ED has been amply evaluated in several pre-clinical trials. A research group has summarized the data27.
Almost all of the studies reported improved erectile function in various animal models of CN injury, vascular insufficiency, diabetes mellitus, hyperlipidemia, and aging. Whilst concerns about the possible promotion of malignant tumors remain whenever stem cells are being transplanted , the use of cell-free solutions like the ANOVA stem cell secretome, the collection of paracrine factors with which stem cells effect repair, has solved this problem, since it avoids the transplantation of the actual stem cells.
Testimonials: Ryan Donovan - erectile dysfunction - BMC Treatment
"I am a 30 year-old man. I have always exercised to keep my body healthy, and I am following a balanced diet. Unfortunately, three years ago I suffered a localized trauma, which gave me some degree of erectile dysfunction. After trying conventional therapies, I contacted ANOVA to try to implement a curative - and not just palliative - approach for my condition. I must thank Mr. Martinovic and the whole ANOVA team, who, from the first contact, handled my case with discreteness and dignity. Dr Sabow, with whom I did the medical consultation and later the procedure, was outstandingly competent and professional. She gave me a realistic description of the results I had to expect from the therapy I chose (BMC for erectile dysfunction). The clinic is modern, and the whole team is full of expertise. The procedure went smoothly, and 3 months later I can enjoy measurable improvements in my condition and in my quality of life."
Ryan Donovan
Are you Interested but Uncertain?
Do you Want a Second Medical Opinion?
Book a Counselling Appointment!
We also offer a service for a second opinion on your current medical records (MRI, CT, X-ray) and treatment advice. Our patient care managers are happy to inform you about what information we need upfront, how to transfer large data files and schedule a counseling appointment with our physicians for you. Please use our contact form to support a fast processing of your case and request.
You are also always welcome to send us an e-mail about your case. The counseling appointment may also take place per telephone or video chat if you live outside Germany. For more intense counseling or additional diagnostic evaluations you may also book an on-site appointment. We can perform needed MRI on the same day. All services rendered by our patient care team are free of charge and we inform you about all physician appointment charges up-front.
Targeting the Cause of ED With Stem Cell Therapy
Despite the wide range of causes of ED, the common denominator is that until now there were only ways to treat the symptoms. Typical medications to treat Erectile Dysfunction (ED) and impotency are based on a substance group called “PDE-5 inhibitors”. Many other drugs have displayed temporal help against impotence in form of injections or creams. There is no clear answer on which of these ED treatment drugs is the best; as they have different properties and side effects. However, all of these drugs have one thing in common: they do not target the cause and therefore are not a “cure”. Their mode of action is based on temporarily overriding the symptoms of weak or no erections.
Regenerative medicine, and the use stem cells as one of their major tools in treating numerous conditions, allows us for a fundamentally different treatment approach. All causes of erectile dysfunction, except in the case of psychologically induced impotence, have some form of primary or secondary blood or nerve supply problem as their main cause. Stem cell-based regenerative therapies have the potential to promote both, angiogenesis (new growth of blood vessels and blood supply improvement) as well as neuro-trophy / neuro-protection (new growth and protection of nerve supply of the penis).
While there are some causes of ED which cannot be treated (i.e. psychologically induced ED), new discoveries in regenerative medicine and stem cells may be able to find the solution. However, it should be noted that a therapy success cannot be guaranteed with any type of treatment. Particularly in the case of experimental treatments such as stem cell therapy, the attending physician is obliged to perform a benefit-to-risk analysis for each patient and determine both the benefits and the risks for that particular patient. If the potential benefits outweigh the potential side effects, they may recommend experimental therapy. Find out about your therapeutic options at ANOVA - call us today.
Personalized Erectile Dysfunction Treatment at ANOVA IRM
Reduction of potency, impotence and erectile dysfunction has a wide range of possible causes. A good medical assessment and diagnostic is absolutely essential before creating a personalized treatment plan for your erectile dysfunction. Seldom will a single injection of random stem cells adequately address your erectile problems. ANOVA is in close cooperation with a fully equipped image diagnostic clinic with several MRI scanners, CT, ultrasound and state of the art blood test and doctors of many specialties. We will analyze the cause of your potency or ED problem and create a treatment plan which is optimized for your problem. Established guideline methods, medications, nutrition and life style recommendations will be included in your treatment plan – together with state of the art and safe stem cell-based therapies.
Frequently Asked Questions: Stem Cell-based Treatments and Regenerative Therapies for Erectile Dysfunction
What is Erectile Dysfunction / Impotence?
Erectile Dysfunctions (ED) is defined as the reduction of potency and libido. All age groups of men can suffer from weak penile erections, which renders their sexual intercourse problematic or even impossible.
What Causes Erectile Dysfunction - ED?
It can have a variety of causes, both physical and psychological. Most frequent reasons are diabetes, smoking, obesity or high blood pressure. Additionally, the effects of aging are partly responsible for impotence and ED.
How is Erectile Dysfunction - ED treated? What Medication is Used to Treat ED?
In the previous decade, the so called “PDE-5 inhibitors” medications were shown to be a successful group of drugs that improved the quality of life of many affected men. However, they offer limited help by temporarily overriding the symptoms of impotence and ED. With recent advancements in stem cell research, promising treatment options have emerged. We at ANOVA, a German Stem Cell Clinic, are the first in Europe to introduce a novel stem cell-based medical treatment approach to treat impotence: The ANOVA Stem Cell Secretome. Schedule an appointment today for more information on how we treat ED with stem cell-based therapies.
What are Stem Cells?
Stem cells are cells that possess the ability to divide into many subgroups of cells, and are responsible for many controlling functions in the body through their paracrine communication (growth factors, hormones, cytokines, anti-inflammatory factors, extracellular vesicles, exosomes and microRNA). We have a full length article describing some of the medically relevant properties of stem cells and stem cell derived treatments here.
Has Research on Stem Cells and ED Been Performed?
Over 20 pre-clinical studies have been published so far on different stem cell models and different damage models in animals for ED since 2004. In 2016, two Phase I study results were published for Bone Marrow Concentrate (BMC) and fat derived Mesenchymal Stem Cells (adMSCs). Both the human and animal studies universally showed an improvement with different interpretations of the results. A breakthrough study was published by Albersen et al. in 2010 that showed that the effects of stem cell injections cannot be accounted to direct differentiation and adherence of the injected stem cells, but rather from their paracrine substances.
Does Stem Cell Therapy Work for Erectile Dysfunction - ED?
The ANOVA Stem Cell Secretome Therapy as the next generation of stem cell-based therapies entails paracrine substances that have the potential to stimulate regeneration. It thus offers a new approach for the treatment of erectile dysfunction and impotence. We are the first in Europe to take full advantage of the stem cells and harness their regenerative powers via their secreted bio-compounds. With the ANOVA Secretome Therapy in combination with Bone Marrow Concentrate Stem Cells we are able to provide a safe way to use stem cells as a natural, personally tailored, regenerative therapy for the different underlying pathologies of erectile dysfunction and impotence.
References and Literature - Stem Cell-based Therapies and Erectile Dysfunction and Impotence
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61.
- Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159–166.
- Chowdhury SH, Cozma AI, Chowdhury JH. Erectile Dysfunction. Essentials for the Canadian Medical Liscensing Exam: Review and Prep for MCCQE Part I. 2nd edition. Wolters Kluwer. Hong Kong. 2017.
- Lue TF, Lee KL. Pharmacotherapy for erectile dysfunction. Chin Med J (Engl) 2000;113:291–298.
- Dorsey P, Keel C, Klavens M, Hellstrom WJ. Phosphodiesterase type 5 (PDE5) inhibitors for the treatment of erectile dysfunction. Expert Opin Pharmacother. 2010;11:1109–1122.
Alwaal A, Hussein AA, Lin CS, Lue TF. Prospects of stem cell treatment in benign urological diseases. Korean J Urol. 2015;56:257–265. - Soebadi MA, Moris L, Castiglione F, Weyne E, Albersen M. Advances in stem cell research for the treatment of male sexual dysfunctions. Curr Opin Urol. 2016;26:129–139.
- Gur, Serap, et al. "Advances in stem cell therapy for erectile dysfunction." Expert opinion on biological therapy just-accepted (2018).
- Panda, Arabind. "Stem cell in urology—are we at the cusp of a new era?." Translational Andrology and Urology (2018).
- https://en.wikipedia.org/wiki/Erection Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013.
- Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813.
- Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20:17–29.
- Lin CS, Xin ZC, Wang Z, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21:343–351.
- Mulhall JP, Bella AJ, Briganti A, et al. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010;7:1687–1698.
- Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005;173:1673–1676.
- Fode M, Ohl DA, et al. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int. 2013;112:998–1008.
- Dashwood MR, Crump A, Shi-Wen X, et al. Identification of neuronal nitric oxide synthase (nNOS) in human penis: a potential role of reduced neuronally-derived nitric oxide in erectile dysfunction. Curr Pharm Biotechnol. 2011;12:1316–1321.
- Gratzke C, Angulo J, Chitaley K, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7:445–475.
- Huang YC, Ning H, Shindel AW, et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391–1400.
- Chen S, Liu Z, Tian N, et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552–556.
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882.
- Pal R, Venkataramana NK, Bansal A, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. 2009;11:897–911.
- Venkataramana NK, Kumar SK, Balaraju S, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res. 2010;155:62–70.
- Violaine K. Harris, James Stark, Tamara Vyshkina, et al. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. EBioMedicine by The Lancet. March 2018 Volume 29, Pages 23–30.
- Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432.
- Dash NR, Dash SN, Routray P, et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359–366.
- Wakitani S, Okabe T, Horibe S, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146–150.
- Khera M, Albersen M, et al. Mesenchymal stem cell therapy for the treatment of erectile dysfunction. J Sex Med. 2015;12:1105–1106.
- Soebadi MA, Moris L, Castiglione F, et al. Advances in stem cell research for the treatment of male sexual dysfunctions. Curr Opin Urol. 2016;26:129–139.
- Zhang H, Yang R, Wang Z, et al. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437–446.
- Albersen M, Lin CS, Lue T. Stem-cell therapy for erectile dysfunction. Arab J Urol. 2013;11:237–244.
- Qiu X, Villalta J, Ferretti L, et al. Effects of intravenous injection of adipose-derived stem cells in a rat model of radiation therapy-induced erectile dysfunction. J Sex Med. 2012;9:1834–1841.
- Alwaal A, Hussein AA, Lin CS, Lue TF. Prospects of stem cell treatment in benign urological diseases. Korean J Urol. 2015;56:257–265.
- You D, Jang MJ, Lee J, et al. Periprostatic implantation of human bone marrow-derived mesenchymal stem cells potentiates recovery of erectile function by intracavernosal injection in a rat model of cavernous nerve injury. Urology. 2013;81:104–110.
- You D, Jang MJ, Lee J, et al. Comparative analysis of periprostatic implantation and intracavernosal injection of human adipose tissue-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Prostate. 2013;73:278–286.
- Lin CS, Xin Z, Dai J, Huang YC, Lue TF. Stem-cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2013;13:1585–1597.
- Lin G, Yang R, Banie L, et al. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010;70:1066–1073.
- Bahk JY, Jung JH, Han H, et al. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant. 2010;8:150–160.
- Yiou R, Hamidou L, Birebent B, et al. Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction: an open dose-escalation pilot study. Eur Urol. 2016;69:988–991.
- Haahr MK, Jensen CH, Toyserkani NM, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. EBioMedicine. 2016;5:204–210.
- Levy JA, Marchand M, Iorio L, et al. Determining the feasibility of managing erectile dysfunction in humans with placental-derived stem cells. J Am Osteopath Assoc. 2016;116:e1–e5.
- Fandel TM, Albersen M, Lin G, et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61:201–210.
- Takayanagi A, Sasaki M, Kataoka-Sasaki Y, et al. Intravenous preload of mesenchymal stem cells rescues erectile function in a rat model of cavernous nerve injury. J Sex Med. 2015;12:1713–1721.
- Vakalopoulos, Ioannis, et al. "Stem cell therapy in erectile dysfunction: science fiction or realistic treatment option?." Hormones (2018): 1-6.
- Wu, Han, et al. "Nanotechnology-assisted adipose-derived stem cell (ADSC) therapy for erectile dysfunction of cavernous nerve injury: In vivo cell tracking, optimized injection dosage, and functional evaluation." Asian journal of andrology 20.5 (2018): 442.
- Matz, Ethan L., et al. "Stem Cell Therapy for Erectile Dysfunction." Sexual medicine reviews (2018).
- Shan, H., Chen, F., Zhang, T., He, S., Xu, L., & Wei, A. (2015). Stem cell therapy for erectile dysfunction of cavernous nerve injury rats: A systematic review and meta-analysis. PLoS ONE, 10(4), 1–23. http://doi.org/10.1371/journal.pone.0121428
- You, Dalsan, et al. "Comparative Study of Autologous Stromal Vascular Fraction and Adipose‐Derived Stem Cells for Erectile Function Recovery in a Rat Model of Cavernous Nerve Injury." Stem cells translational medicine 4.4 (2015): 351-358.
- Chen, X., Yang, Q., Zheng, T., Bian, J., Sun, X., Shi, Y., … Deng, C. (2016). Neurotrophic Effect of Adipose Tissue-Derived Stem Cells on Erectile Function Recovery by Pigment Epithelium-Derived Factor Secretion in a Rat Model of Cavernous Nerve Injury, 2016.
- Yiou, R., Hamidou, L., Birebent, B., Bitari, D., Lecorvoisier, P., Contremoulins, I., … Rouard, H. (2016). Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postradical Prostatectomy Erectile Dysfunction: An Open Dose-Escalation Pilot Study. European Urology, 69(6), 988–991. http://doi.org/10.1016/j.eururo.2015.09.026
- Wang, X. Y., Liu, C. L., Li, S. D., Xu, Y., Chen, P., Liu, Y., … Yang, M. H. (2015). Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PLoS ONE, 10(3), 1–18. http://doi.org/10.1371/journal.pone.0118951
- Haahr, M. K., Jensen, C. H., Toyserkani, N. M., Andersen, D. C., Damkier, P., S??rensen, J. A., … Sheikh, S. P. (2016). Safety and Potential Effect of a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. EBioMedicine, 5, 204–210. http://doi.org/10.1016/j.ebiom.2016.01.024
- Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, et al. (2004) The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. Bju Int 94: 904–09. PMID: 15476533
- Kim Y, de Miguel F, Usiene I, Rahman N, Yoshimura N, Huard J, et al. (2006) Injection of skeletal muscle-derived cells into the penis improves erectile function. Int J Impot Res 18: 329–34. PMID: 16341028
- Fall PA, Izikki M, Tu L, Swieb S, Giuliano F, Bernabe J, et al. (2009) Apoptosis and effects of intracavernous bone marrow cell injection in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2009; 56: 716–25. doi: 10.1016/j.eururo.2008.09.059 PMID: 18922625
- Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, et al. (2010) Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med 7: 3331–40. doi: 10.1111/j.1743-6109.2010.01875.x PMID: 20561166
- Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ, Spees JL (2010) Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol 184: 1560–66. doi: 10.1016/j.juro.2010.05.088 PMID: 20728109
- Lin G, Qiu X, Fandel T, Banie L, Wang G, Lue TF, et al. (2011) Tracking intracavernously injected adipose-derived stem cells to bone marrow. Int J Impot Res 23: 268–75. doi: 10.1038/ijir.2011.38 PMID: 21796145
- Lin G, Albersen M, Harraz AM, Fandel TM, Garcia M, McGrath MH, et al. (2011) Cavernous nerve repair with allogenic adipose matrix and autologous adipose-derived stem cells. Urology 77: 1501–09. Woo JC, Bae WJ, Kim SJ, Kim SD, Sohn DW, Hong SH, et al. (2011) Transplantation of muscle-derived stem cells into the corpus cavernosum restores erectile function in a rat model of cavernous nerve injury.
- Korean J Urol 52: 359–63. doi: 10.4111/kju.2011.52.5.359 PMID: 21687398 Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, et al. (2012) Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol 61: 201–10. doi: 10.1016/j.eururo.2011.07.061 PMID: 21824718
- Kim SJ, Park SH, Sung YC, Kim SW (2012) Effect of mesenchymal stem associated to matrixen on the erectile function in the rat model with bilateral cavernous nerve crushing injury. Int Braz J Urol 38: 833– 41. PMID: 23302404
- Kim SJ, Choi SW, Hur KJ, Park SH, Sung YC, Ha YS, et al. (2012) Synergistic effect of mesenchymal stem cells infected with recombinant adenovirus expressing human BDNF on erectile function in a rat model of cavernous nerve injury. Korean J Urol 53: 726–32. doi: 10.4111/kju.2012.53.10.726 PMID: 23136635
- Kovanecz I, Rivera S, Nolazco G, Vernet D, Segura D, Gharib S, et al. (2012) Separate or combined treatments with daily sildenafil, molsidomine, or muscle-derived stem cells prevent erectile dysfunction in a rat model of cavernosal nerve damage. J Sex Med 9: 2814–26. doi: 10.1111/j.1743-6109.2012. 02913.x PMID: 22974131
- Piao S, Kim IG, Lee JY, Hong SH, Kim SW, Hwang TK, et al. (2012) Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J Sex Med 9: 1968–79. doi: 10.1111/j.1743-6109.2012.02760.x PMID: 22642440
- Qiu X, Villalta J, Ferretti L, Fandel TM, Albersen M, Lin G, et al. (2012) Effects of intravenous injection of adipose-derived stem cells in a rat model of radiation therapy-induced erectile dysfunction. J Sex Med 9: 1834–41. doi: 10.1111/j.1743-6109.2012.02753.x PMID: 22548750
- Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, et al. (2010) Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med 7: 3331–40. doi: 10.1111/j.1743-6109.2010.01875.x PMID: 20561166
- Tewari, A., et al. "Technique of traction-free nerve-sparing robotic prostatectomy: delicate tissue handling by real-time penile oxygen monitoring." International journal of impotence research 24.1 (2012): 11.
Further References for MSC, BMC, Stemcell Secretome and EVs
- Georg Hansmann, Philippe Chouvarine, Franziska Diekmann, Martin Giera, Markus Ralser, Michael Mülleder, Constantin von Kaisenberg, Harald Bertram, Ekaterina Legchenko & Ralf Hass "Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension". Nature Cardiovascular Research volume 1, pages568–576 (2022).
- Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis . Arthritis Rheum. 2003;48:3464–74.
- Lee KB, Hui JH, Song IC, Ardany L, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cell. 2007;25:2964–71.
- Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy . 2009;25(12):1391–400.
- Black L, Gaynor J, Adams C, et al. Effect of intra-articular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200.
- Centeno C, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–53.
- Centeno C, Kisiday J, Freeman M, et al. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: a case study. Pain Physician. 2006;9:253–6.
- Centeno C, Schultz J, Cheever M. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell. 2011;5(1):81–93.
- Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose derived stem cells: a case series. J Med Case Rep. 2001;5:296.
- Kuroda R, Ishida K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31.
- Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422–8.
- Saw KY et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94.
- Vangsness CT, Farr J, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg. 2014;96(2):90–8.
- Freitag, Julien, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy–a review. BMC musculoskeletal disorders 17.1 (2016): 230.
- Maumus, Marie, Christian Jorgensen, and Danièle Noël. " Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. " Biochimie 95.12 (2013): 2229-2234.
- Dostert, Gabriel, et al. " How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication?. " Frontiers in Cell and Developmental Biology 5 (2017).
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- Toh, Wei Seong, et al. " MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. " Seminars in Cell & Developmental Biology. Academic Press, 2016.
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- S. Koelling, J. Kruegel, M. Irmer, J.R. Path, B. Sadowski, X. Miro, et al., Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis , Cell Stem Cell 4 (2009) 324–335.
- B.A. Jones, M. Pei, Synovium-Derived stem cells: a tissue-Specific stem cell for cartilage engineering and regeneration , Tissue Eng. B: Rev. 18 (2012) 301–311.
- W. Ando, J.J. Kutcher, R. Krawetz, A. Sen, N. Nakamura, C.B. Frank, et al., Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage, Cytotherapy 16 (2014) 776–788.
- K.B.L. Lee, J.H.P. Hui, I.C. Song, L. Ardany, E.H. Lee, Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model, Stem Cells 25 (2007) 2964–2971.
- W.-L. Fu, C.-Y. Zhou, J.-K. Yu, A new source of mesenchymal stem cells for articular cartilage repair: mSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model , Am. J. Sports Med. 42 (2014) 592–601.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, et al., Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration , Biomaterials 33 (2012) 7008–7018.
- E.-R. Chiang, H.-L. Ma, J.-P. Wang, C.-L. Liu, T.-H. Chen, S.-C. Hung, Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits , PLoS One 11 (2016) e0149835.
- H. Nejadnik, J.H. Hui, E.P. Feng Choong, B.-C. Tai, E.H. Lee, Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study , Am. J. Sports Med. 38 (2010) 1110–1116.
- I. Sekiya, T. Muneta, M. Horie, H. Koga, Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects , Clin. Orthop. Rel. Res. 473 (2015) 2316–2326.
- Y.S. Kim, Y.J. Choi, Y.G. Koh, Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes , Am. J. Sports Med. 43 (2015) 2293–2301.
- W.-L. Fu, Y.-F. Ao, X.-Y. Ke, Z.-Z. Zheng, X. Gong, D. Jiang, et al., Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment , Knee 21 (2014) 609–612.
- Y.-G. Koh, O.-R. Kwon, Y.-S. Kim, Y.-J. Choi, D.-H. Tak, Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial , Arthrosc. J. Arthrosc. Relat. Surg. 32 (2016) 97–109.
- T.S. de Windt, L.A. Vonk, I.C.M. Slaper-Cortenbach, M.P.H. van den Broek, R. Nizak, M.H.P. van Rijen, et al., Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-Stage cartilage repair in humans upon mixture with recycled autologous chondrons , Stem Cells (2016) (n/a-n/a).
- L. da Silva Meirelles, A.M. Fontes, D.T. Covas, A.I. Caplan, Mechanisms involved in the therapeutic properties of mesenchymal stem cells , Cytokine Growth Factor Rev. 20 (2009) 419–427.
- W.S. Toh, C.B. Foldager, M. Pei, J.H.P. Hui, Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration , Stem Cell Rev. Rep. 10 (2014) 686–696.
- R.C. Lai, F. Arslan, M.M. Lee, N.S.K. Sze, A. Choo, T.S. Chen, et al., Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury , Stem Cell Res. 4 (2010) 214–222.
- S. Zhang, W.C. Chu, R.C. Lai, S.K. Lim, J.H.P. Hui, W.S. Toh, Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration, Osteoarthr . Cartil. 24 (2016) 2135–2140.
- S. Zhang, W. Chu, R. Lai, J. Hui, E. Lee, S. Lim, et al., 21 – human mesenchymal stem cell-derived exosomes promote orderly cartilage regeneration in an immunocompetent rat osteochondral defect model , Cytotherapy 18 (2016) S13.
- C.T. Lim, X. Ren, M.H. Afizah, S. Tarigan-Panjaitan, Z. Yang, Y. Wu, et al., Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model
- A. Gobbi, G. Karnatzikos, S.R. Sankineani, One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee , Am. J. Sports Med. 42 (2014) 648–657.
- A. Gobbi, C. Scotti, G. Karnatzikos, A. Mudhigere, M. Castro, G.M. Peretti, One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years , Knee Surg. Sports Traumatol. Arthrosc. (2016) 1–8.
- A. Gobbi, G. Karnatzikos, C. Scotti, V. Mahajan, L. Mazzucco, B. Grigolo, One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-Year follow-up , Cartilage 2 (2011) 286–299.
- K.L. Wong, K.B.L. Lee, B.C. Tai, P. Law, E.H. Lee, J.H.P. Hui, Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up , Arthrosc. J. Arthrosc. Relat. Surg. 29 (2013) 2020–2028.
- J.M. Hare, J.E. Fishman, G. Gerstenblith, et al., Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the poseidon randomized trial, JAMA 308 (2012) 2369–2379.
- L. Wu, J.C.H. Leijten, N. Georgi, J.N. Post, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation , Tissue Eng. A 17 (2011) 1425–1436.
- L. Wu, H.-J. Prins, M.N. Helder, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells in chondrocyte Co-Cultures are independent of culture conditions and cell sources , Tissue Eng. A 18 (2012) 1542–1551.
- S.K. Sze, D.P.V. de Kleijn, R.C. Lai, E. Khia Way Tan, H. Zhao, K.S. Yeo, et al., Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells , Mol. Cell. Proteomics 6 (2007) 1680–1689.
- M.B. Murphy, K. Moncivais, A.I. Caplan, Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine , Exp. Mol. Med. 45 (2013) e54.
- M.J. Lee, J. Kim, M.Y. Kim, Y.-S. Bae, S.H. Ryu, T.G. Lee, et al., Proteomic analysis of tumor necrosis factor--induced secretome of human adipose tissue-derived mesenchymal stem cells , J. Proteome Res. 9 (2010) 1754–1762.
- S. Bruno, C. Grange, M.C. Deregibus, R.A. Calogero, S. Saviozzi, F. Collino, et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury, J. Am. Soc. Nephrol. 20 (2009) 1053–1067.
- M. Yá˜nez-Mó, P.R.-M. Siljander, Z. Andreu, A.B. Zavec, F.E. Borràs, E.I. Buzas, et al. Biological properties of extracellular vesicles and their physiological functions (2015).
- C. Lawson, J.M. Vicencio, D.M. Yellon, S.M. Davidson, Microvesicles and exosomes: new players in metabolic and cardiovascular disease , J. Endocrinol. 228 (2016) R57–R71.
- A.G. Thompson, E. Gray, S.M. Heman-Ackah, I. Mager, K. Talbot, S.E. Andaloussi, et al., Extracellular vesicles in neurodegenerative diseas—pathogenesis to biomarkers, Nat. Rev. Neurol. 12 (2016) 346–357.
- I.E.M. Bank, L. Timmers, C.M. Gijsberts, Y.-N. Zhang, A. Mosterd, J.-W. Wang, et al., The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease , Expert Rev. Mol. Diagn. 15 (2015) 1577–1588.
- T. Kato, S. Miyaki, H. Ishitobi, Y. Nakamura, T. Nakasa, M.K. Lotz, et al., Exosomes from IL-1 stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes , Arthritis. Res. Ther. 16 (2014) 1–11.
- R.W.Y. Yeo, S.K. Lim, Exosomes and their therapeutic applications, in: C. Gunther, A. Hauser, R. Huss (Eds.), Advances in Pharmaceutical Cell TherapyPrinciples of Cell-Based Biopharmaceuticals, World Scientific, Singapore, 2015, pp. 477–491.
- X. Qi, J. Zhang, H. Yuan, Z. Xu, Q. Li, X. Niu, et al., Exosomes secreted by human-Induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats , Int. J. Biol. Sci. 12 (2016) 836–849.
- R.C. Lai, F. Arslan, S.S. Tan, B. Tan, A. Choo, M.M. Lee, et al., Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles , J. Mol. Cell. Cardiol. 48 (2010) 1215–1224.
- Y. Zhou, H. Xu, W. Xu, B. Wang, H. Wu, Y. Tao, et al., Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro , Stem Cell Res. Ther. 4 (2013) 1–13.
- Y. Qin, L. Wang, Z. Gao, G. Chen, C. Zhang, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo , Sci. Rep. 6 (2016) 21961.
- M. Nakano, K. Nagaishi, N. Konari, Y. Saito, T. Chikenji, Y. Mizue, et al., Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes , Sci. Rep. 6 (2016) 24805.
- K. Nagaishi, Y. Mizue, T. Chikenji, M. Otani, M. Nakano, N. Konari, et al., Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes , Sci. Rep. 6 (2016) 34842.
- S.R. Baglio, K. Rooijers, D. Koppers-Lalic, F.J. Verweij, M. Pérez Lanzón, N. Zini, et al., Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species , Stem Cell Res. Ther. 6 (2015) 1–20.
- T. Chen, R. Yeo, F. Arslan, Y. Yin, S. Tan, Efficiency of exosome production correlates inversely with the developmental maturity of MSC donor, J. Stem Cell Res. Ther. 3 (2013) 2.
- R.C. Lai, S.S. Tan, B.J. Teh, S.K. Sze, F. Arslan, D.P. de Kleijn, et al., Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome , Int. J. Proteomics 2012 (2012) 971907.
- T.S. Chen, R.C. Lai, M.M. Lee, A.B.H. Choo, C.N. Lee, S.K. Lim, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs , Nucleic Acids Res. 38 (2010) 215–224.
- R.W. Yeo, R.C. Lai, K.H. Tan, S.K. Lim, Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell, J. Circ. Biomark. 1 (2013) 7.
- R.C. Lai, R.W. Yeo, S.K. Lim, Mesenchymal stem cell exosomes, Semin. Cell Dev. Biol. 40 (2015) 82–88.
- B. Zhang, R.W. Yeo, K.H. Tan, S.K. Lim, Focus on extracellular vesicles: therapeutic potential of stem cell-derived extracellular vesicles , Int. J. Mol. Sci. 17 (2016) 174.
- Hu G-w, Q. Li, X. Niu, B. Hu, J. Liu, Zhou S-m, et al., Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice , Stem Cell Res. Ther. 6 (2015) 1–15.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li, et al., Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis , J. Transl. Med. 13 (2015) 1–14.
- B. Zhang, M. Wang, A. Gong, X. Zhang, X. Wu, Y. Zhu, et al., HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing, Stem Cells 33 (2015) 2158–2168.
- B. Zhang, Y. Yin, R.C. Lai, S.S. Tan, A.B.H. Choo, S.K. Lim, Mesenchymal stem cells secrete immunologically active exosomes , Stem Cells Dev. 23 (2013) 1233–1244.
- C.Y. Tan, R.C. Lai, W. Wong, Y.Y. Dan, S.-K. Lim, H.K. Ho, Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models , Stem Cell Res. Ther. 5 (2014) 1–14.
- C. Lee, S.A. Mitsialis, M. Aslam, S.H. Vitali, E. Vergadi, G. Konstantinou, et al., Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension , Circulation 126 (2012) 2601–2611.
- B. Yu, H. Shao, C. Su, Y. Jiang, X. Chen, L. Bai, et al., Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1 , Sci. Rep. 6 (2016) 34562.
- Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof of concept clinical trial. Stem Cells. 2014;32(5):1254–66.
- Vega, Aurelio, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
- Davatchi F, Sadeghi-Abdollahi B, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–5
- Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case- controlled study. Int Orthop. 2014;38(9):1811–1818
- Galli D, Vitale M, Vaccarezza M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:6.
- Beitzel K, Solovyova O, Cote MP, et al. The future role of mesenchymal Stem cells in The management of shoulder disorders . Arthroscopy. 2013;29(10):1702–1711.
- Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190.
- Malda, Jos, et al. " Extracellular vesicles [mdash] new tool for joint repair and regeneration. " Nature Reviews Rheumatology (2016).
Further References about PRP
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4(1):52–62.
Extras
- Xu, Ming, et al. " Transplanted senescent cells induce an osteoarthritis-like condition in mice. " The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (2016): glw154.
- McCulloch, Kendal, Gary J. Litherland, and Taranjit Singh Rai. " Cellular senescence in osteoarthritis pathology ." Aging Cell (2017).
Patient Services at ANOVA Institute for Regenerative Medicine
- Located in the center of Germany, quick access by car or train from anywhere in Europe
- Simple access worldwide, less than 20 minutes from Frankfurt Airport
- Individualized therapy with state-of-the-art stem cell products
- Individually planned diagnostic work-up which include world-class MRI and CT scans
- German high quality standard on safety and quality assurance
- Personal service with friendly, dedicated Patient Care Managers
- Scientific collaborations with academic institutions to assure you the latest regenerative medical programs