Counteract the Causes and Damaging Effects of Dry Mouth Syndrome
with Targeted Stem Cell-based Injections in Saliva Glands
Dry mouth syndrome comes with a variety of undesirable conditions. As a consequence of insufficient wetting, impairment of oral functions such as speech, chewing, and swallowing, as well as a reduced lubrication of consumed foods is possible. Additionally, the oral mucosa can regularly be affected by ulceration, frictional trauma, while the mouth in general can be prone to excessive microbial overgrowth, leading to dental erosion, caries and oral candidiasis.
Systemic disease such as high blood pressure and diabetes, and their management through medication can cause dry mouth symptoms. There are also autoimmune causes, like Lupus, Sjögrens, sarcoidosis or chronic eczema. Besides these factors, a wide range of other diseases can result in dry mouth symptoms.
Besides the numerous medical therapies which can significantly decrease salivary gland function, the most severe cases are due to radiation therapy or chemotherapy in Head and neck cancer patients. The incidence of Head and Neck Cancer is globally increasing, with approx. 300.000 of newly diagnosed cases every year. Radiation therapy as one of the most important treatments in Head and Neck Cancer significantly affects healthy tissue which surrounds the tumor, which usually includes the salivary glands. The decline of gland function leading to dry mouth is called “xerostomia”, and often leads to the oral symptoms described above, which has a severe negative impact on quality of life of the patients.
Dry Mouth Syndrome - Diagnostics - Treatment - Stem Cells - Medication
On this page, we inform you about Dry Mouth Syndrome giving an overview on important aspects of causes, treatment options, precision diagnostics that reveals the cause of pain as well as our stem cell-based therapies that we offer in Offenbach (near Frankfurt am Main) Germany.
Jump directly to the following topics:
- Conventional therapies
- ANOVA therapies for Dry Mouth
- Expectations and limits
- Diagnostics of inflammatory sites
- The ANOVA difference: targeted treatment
- You want a second opinion
- Workflow of the treatment process
- FAQ- frequently-asked questions
- Sources and Literature
Existing Conventional Therapies for Dry Mouth Syndrome
Medication, Lubricants and Time
Currently available treatments aim at improving salivary gland function, but all of them are symptomatic treatments. This means that those strategies merely stimulate the function of the remaining salivary gland tissue, or they provide limited improvement in lubrication. Some of them include sialagogues, saliva stimulants (such as sugar-free chewing gum and candy with weak acidity), and the application of saliva substitutes and oral lubricants. All of these bring short-term ease, but none of them regenerate the tissue for a long-term effect.
Usually, symptoms decrease with time and after 2-3 years, saliva productions slowly increases.
Stem Cell Treatments for Dry Mouth Syndrome - DMS at
ANOVA Institute for Regenerative Medicine - Offenbach, Germany
BMC, Secretome/Exosomes, PRP
ANOVA Institute for Regenerative Medicine is now offering stem cell treatment for patients with Dry Mouth syndrome. Get in touch with our medical professionals today to find out more about your treatment options.
Potency Hypothesis of Stem Cell Therapies
Stem cells possess the potential to communicate with the immune cells that elicit inflammation and by natural, so far not understood mechanisms may inhibit the immune-over-reaction that blocks regeneration. Furthermore, stem cells have the ability to stimulate regeneration of tissue thereby fasten the tissue recovery. The aim of a stem cell treatment for DMS is to reduce residual inflammation and to stimulate and fasten regeneration in radiation-damaged saliva glands.
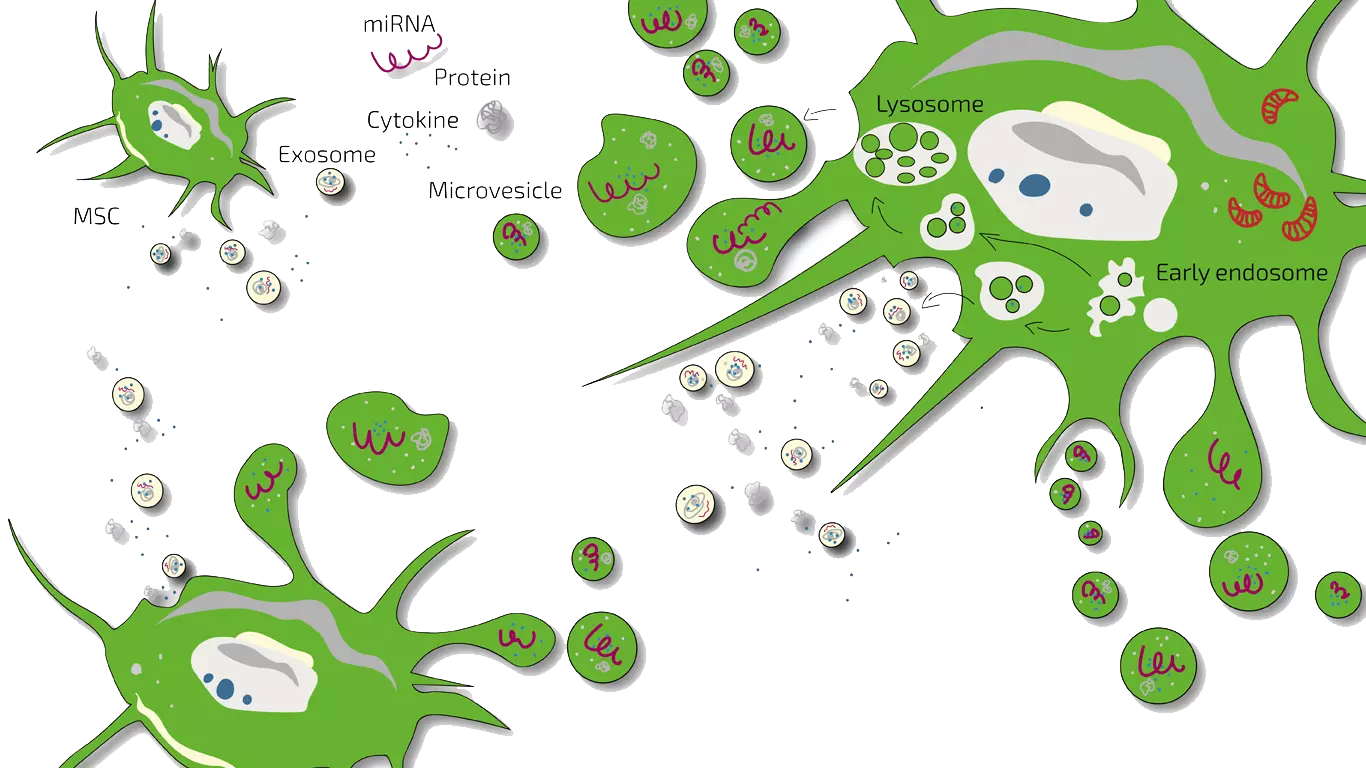
MSEC - Mesenchymal Stem Cell Secretome - Exosomes - Autologous
As Dry Mouth Syndrome arises from severe damage to salivary glands, we usually treat patients with MSEC (secretome, exosomes, EVs) of mesenchymal stem cells (MSC, AD-MSC, adipose-derived, fat-derived stem cells) which we harvest from the patients belly in a mini-liposuction (very brief and limited liposuction) under slight sedation. Worldwide, ANOVA is the first stem cell clinic to acquire legal permission form the responsible governmental authorities and therefore, offers high quality, safe and legally-controlled autologous (own) exosome-containing secretome.
The main advantage of MSEC is that in contrast to live stem cells which would loose their therapeutic potency, can be frozen without loss of exosomes. This enables us to produce 10-20 injection doses from one liposuction which can then be administered over a longer treatment period. This is especially advantageous for continuous stimulation of saliva gland regeneration in DMS. The MSEC can be injected directly into the damaged glands.
What a Secretome/Exosome is and how they compare is explained on our overview page.
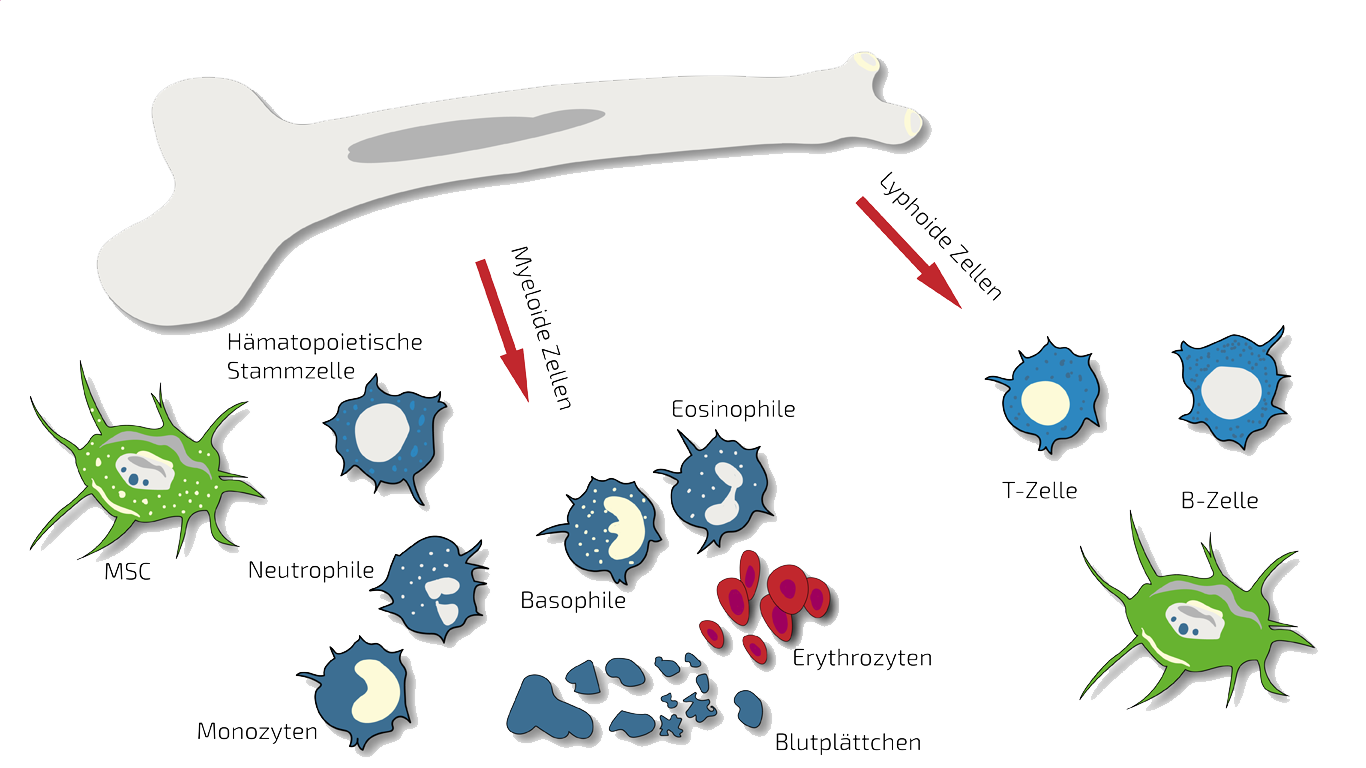
BMC - Bone Marrow Concentrate - Autologous
Autologous (self) BMC are the second stem cell therapy that we use for treatment of dry mouth syndrome. However, BMC is a one-injection per bone marrow donation treatment.
In severe cases, we initiate treatment with localized BMC injection followed by localized MSEC injections. BMC contains autologous meaning patients own, adult stem cells (hematopoietic and mesenchymal stem cells in natural composition) which we isolate and concentrate from your pelvis crest in a short process under slight sedation.
These stem cells are supposed to inhibit the residual inflammation and stimulate the regeneration of saliva glands.
More information about this type of stem cell therapy is summarized on our page an BMC.
PRP - Platelet-Rich Plasma - Autologous
PRP - platelet-rich plasma is usually not used in the treatment of Dry Mouth Syndrome.
It is a comparatively inexpensive, blood-derived stimulant for wound healing and repair.
More on PRP (as a combination therapy) is summarized on our PRP overview page.
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
How does the ANOVA Therapy differ?
Diagnostics – We Look for the Cause of your Pain
Dr. mult. Michael K. Stehling, the founder of ANOVA IRM and the Vitus Prostate Center , is a radiologist (MD) and holds a PhD in physics. For this reason, the ANOVA Institute for Regenerative Medicine, in cooperation with the Prof. Stehling Institute for Diagnostic Imaging located in the same building, has the capability to use special precision diagnostics such as arthro-MRI and non-radioactive contrast MRIs.
Compared to many conventional MRIs, these methods are often able to localize the pain-causing inflammation in your joints and tissues. This enables us to determine individually how patients should be treated and where the stem cells should be applied.
Furthermore, in consultation with you and if advisable, we supplement our patient-specific diagnostics with specific blood tests on hormones, inflammation parameters and other factors that are important in your case, or recommend further examinations such as a preventive MRI spinal scan.
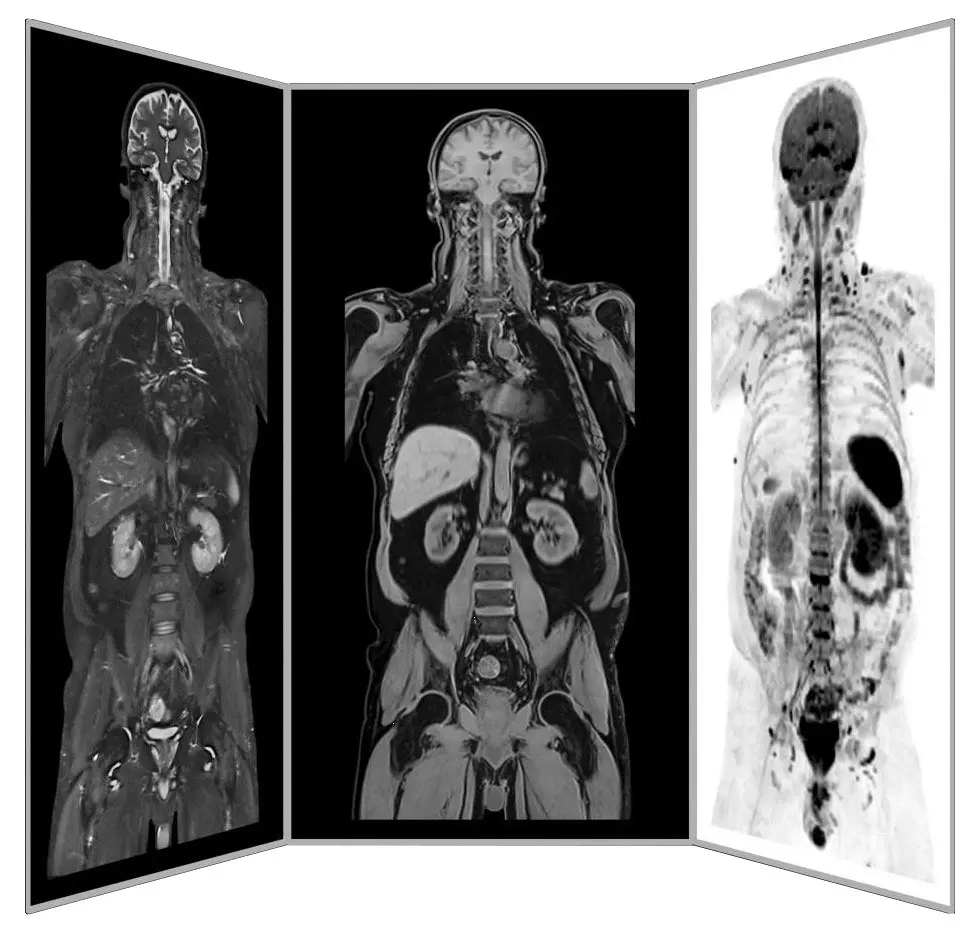
Precision MRI scans -
find the source of pain
ANOVA IRM © Siemens Healthcare GmbH
How Does the ANOVA Therapy Differ?
We Implant the Stem Cells Precisely Where They are Needed
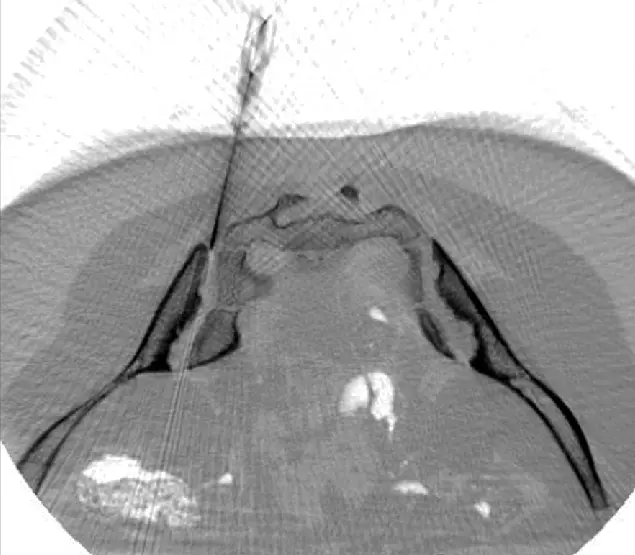
CT-assisted stem cell injection into joints
ANOVA IRM © Siemens Healthcare GmbH
Based on our specific diagnostics using arthro-MRI and non-radioactive contrast medium MRIs, we can, in contrast to many other clinics, deliver the stem cells with image support, e.g. using CT, precisely to the affected area. This means we can inject into and at glands to specifically and quickly trigger an effect where regeneration is to be induced. All interventions are performed under supervision and care of our anesthesiologist and are pain free.
A purely intravenous administration, as many other clinics do, is only performed for the secretome (exosomes) if it is needed to centrally modulate immune reactions.
Of course, we will thoroughly advise you in the early process and the on-site consultation in advance on all steps and discuss alternatives and expectations.
Are you Interested but Uncertain? Book a Counseling Appointment!
Our patient care managers are happy to inform you about what information we need upfront (MRI, CT, X-ray), how to transfer large data files and schedule a counseling appointment with our physicians for you. Our patient care managers are happy to inform you about what information we need upfront, how to transfer large data files and schedule a counseling appointment with our physicians for you. Please use our contact form to support a fast processing of your case and request.
You are also always welcome to send us an e-mail about your case. The counseling appointment may also take place per telephone or video chat if you live outside Germany. For more intense counseling or additional diagnostic evaluations you may also book an on-site appointment. We can perform needed MRI on the same day. All services rendered by our patient care team are free of charge and we inform you about all physician appointment charges up-front.
Therapy Workflow for Dry Mouth Syndrome
The precise workflow is described in detail on the stem cell- specific pages of BMC, Secretome/Exosomes and PRP (rarely used in DMS cases).
All therapies are divided into phases such as evaluation of the medical history (we analyze your current therapies and medical records), initial counseling and evaluation of potential, patient-individual benefit of a stem cell therapy (indication statement), preliminary examinations, diagnostics, consultation on all therapy options, preparation of an individual treatment plan including cost estimate, harvesting of tissue, production of the stem cell product, quality control of the product and application. There are two special features for osteoarthritis and arthritis patients. If your previous findings have not found the specific causes of your joint pain, we will examine you in advance with a precise and informative arthro-MRI or an MRI with non-radioactive contrast medium, if you wish. In addition, we often apply the stem cells (BMC) intra-articularly (i.e., directly in the joint). This means that we deliver the stem cells to the exact location where your pain originates.
Unfortunately, according to the risk-benefit ratio, we cannot treat children or pregnant women. In addition, other factors can also be exclusion criteria.
How Long Does a Stem Cell Therapy Take?
The initial analyses and counselling can be done without you having to travel to Offenbach (near Frankfurt/Main, Germany). This period can be 2 weeks up to months depending on the availability of patients slots. If you live further away, we will conduct the initial discussions by telephone or video conference. For the actual treatment, you will travel to Offenbach. Then, depending on the therapy, the tissue collection, quality control and treatment type it will take as follows:
BMC- and PRP-Therapy
Each donation and application of BMC and PRP (as combination therapy) on-site period: 2 days (consecutive days).
Secretome/Exosome-Therapy:
Preparation and harvest of the fat (mini-liposuction) need once 2 days (consecutive days) in Offenbach, followed by enrichment of the mesenchymal stem cells (Secretome/Exosome) and quality control. Approximately 4 weeks after the isolation, the therapy begins according to the therapy plan determined with you. You will then come to Offenbach am Main (Germany) several times for the application. The shelf life of the secretome (exosomes) is 2 years.
How Much Does Stem Cell Treatment Cost?
Our treatments are always tailored to your specific situation, disease, stage and other factors. The therapies differ in the product used (BMC, secretome or PRP), the frequency of treatment as well as the further examinations and your sedation and anesthesia wishes. A treatment for Dry Mouth Syndrome can cost from a few hundred to several thousand euros. You will receive a cost estimate for all treatments in advance so that you can accurately estimate what a treatment would cost in your individual case.
Does my Health Insurance Cover the Therapy Costs?
Unfortunately, at the moment it is assumed that health insurance companies do not cover the costs of experimental therapies (BMC, secretome, PRP, micro-fracture technique), i.e. you will have to bear the costs entirely yourself.
The Power of Stem Cells Treatment - External Scientific results
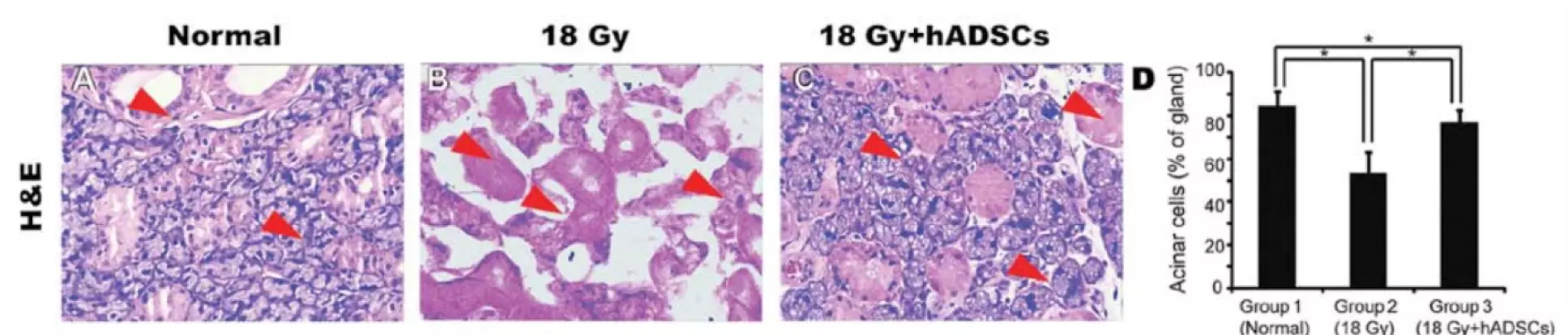
Salivary gland tissue restoration after injury by human adipose tissue-derived stem cells (hADSCs). Shown are H&E stained tissue samples. A. Control sample of a normal rat submandibular salivary gland. Visible is mixed glandular tissue. B. Tissue sample after radiation therapy at 18 Gy. Visible is coagulative necrosis and interstitial edema at 24 weeks. C. hADSC-treated cells of the salivary glands after treatment at 18 Gy. Tissue sample shows significant regeneration, with a greater number of normal tissue. D. Mean percentage of acinar cells in the salivary gland. Transplanted stem cells significantly increased acinar cells in irradiated tissue. © Xiong et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY_NC 3.0].1
Adult stem cells are abundant in almost all tissues. Stem cells have been identified as a resourceful option for the treatment of a wide range of degenerative disorders. Their effectiveness is known to lie in their secreted molecules, as well as in their ability to differentiate into different types of specialized cells. Recent research has shown that mesenchymal stem cells (MSCs), and in particular stem cell secretome Therapy have a number of characteristics which are useful in tissue regeneration, including anti-inflammatory and anti-apoptotic effects. Stem cells can enhance immunological parameters and tissue-regenerating properties. For radiation-induced xerostomia (which is dry mouth due to cancer radiation treatment), stem cells have shown promising results in recent studies 1,2. The results indicate that stem cells improve the amount of saliva by 33% after four weeks and 50% after four months 2. Additionally, stem cells may reduce the amount of scar/fibrous tissue in the gland and the feeling of thirst.2
Please note that stem cell therapy is still an experimental therapy for all medical indications, including dry mouth. Therefore, your attending physician will assess the benefits and risks of stem cell-based therapy for your individual case. If the benefits outweigh the risks, your doctor can suggest the treatment as an option to you.
Frequently Asked Questions: Stem Cell-Based Treatments and Regenerative Therapies for Osteoarthritis
What is Dry Mouth Syndrome?
Dry mouth syndrome (Xerostomia) is the symptom-based name of a condition in which the salivary glands in the mouth are not able or do not produce sufficient saliva to keep the mouth and tongue wet. Dry mouth often arises as a side effect of radiation therapy for cancer or certain medications. In rare cases it seems to be a aging-related issue.
How is Dry Mouth Treated?
The normal treatments follow the following strategies:
- Reduce all substances that can further reduce saliva: alcohol, tobacco, caffeine, mouth washes unless DMS-approved
- Reduce drying from breathing: enhanced breathing through the nose
- Stimulate saliva production: sugar-free chewing gum, sweets
- Passive wetting: frequent zips of water or herbal tee, medical lubricant spays
Sources and Literature
- Xiong, X., Shi, X., & Chen, F. (2014). Human adipose tissue derived stem cells alleviate radiation induced xerostomia. International journal of molecular medicine, 34(3), 749-755.
- Grønhøj, C., Jensen, D. H., Vester-Glowinski, P., Jensen, S. B., Bardow, A., Oliveri, R. S., ... & Jensen, M. (2018). Safety and efficacy of mesenchymal stem cells for radiation-induced xerostomia: a randomized, placebo-controlled phase 1/2 trial (MESRIX). International Journal of Radiation Oncology* Biology* Physics, 101(3), 581-592.
Further References for MSC, BMC, Stemcell Secretome and EVs
- Georg Hansmann, Philippe Chouvarine, Franziska Diekmann, Martin Giera, Markus Ralser, Michael Mülleder, Constantin von Kaisenberg, Harald Bertram, Ekaterina Legchenko & Ralf Hass "Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension". Nature Cardiovascular Research volume 1, pages568–576 (2022).
- Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis . Arthritis Rheum. 2003;48:3464–74.
- Lee KB, Hui JH, Song IC, Ardany L, et al. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cell. 2007;25:2964–71.
- Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy . 2009;25(12):1391–400.
- Black L, Gaynor J, Adams C, et al. Effect of intra-articular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200.
- Centeno C, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–53.
- Centeno C, Kisiday J, Freeman M, et al. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: a case study. Pain Physician. 2006;9:253–6.
- Centeno C, Schultz J, Cheever M. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell. 2011;5(1):81–93.
- Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose derived stem cells: a case series. J Med Case Rep. 2001;5:296.
- Kuroda R, Ishida K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31.
- Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422–8.
- Saw KY et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94.
- Vangsness CT, Farr J, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg. 2014;96(2):90–8.
- Freitag, Julien, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy–a review. BMC musculoskeletal disorders 17.1 (2016): 230.
- Maumus, Marie, Christian Jorgensen, and Danièle Noël. " Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. " Biochimie 95.12 (2013): 2229-2234.
- Dostert, Gabriel, et al. " How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication?. " Frontiers in Cell and Developmental Biology 5 (2017).
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- Toh, Wei Seong, et al. " MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. " Seminars in Cell & Developmental Biology. Academic Press, 2016.
- Chaparro, Orlando, and Itali Linero. " Regenerative Medicine: A New Paradigm in Bone Regeneration. " (2016).
- S. Koelling, J. Kruegel, M. Irmer, J.R. Path, B. Sadowski, X. Miro, et al., Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis , Cell Stem Cell 4 (2009) 324–335.
- B.A. Jones, M. Pei, Synovium-Derived stem cells: a tissue-Specific stem cell for cartilage engineering and regeneration , Tissue Eng. B: Rev. 18 (2012) 301–311.
- W. Ando, J.J. Kutcher, R. Krawetz, A. Sen, N. Nakamura, C.B. Frank, et al., Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage, Cytotherapy 16 (2014) 776–788.
- K.B.L. Lee, J.H.P. Hui, I.C. Song, L. Ardany, E.H. Lee, Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model, Stem Cells 25 (2007) 2964–2971.
- W.-L. Fu, C.-Y. Zhou, J.-K. Yu, A new source of mesenchymal stem cells for articular cartilage repair: mSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model , Am. J. Sports Med. 42 (2014) 592–601.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, et al., Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration , Biomaterials 33 (2012) 7008–7018.
- E.-R. Chiang, H.-L. Ma, J.-P. Wang, C.-L. Liu, T.-H. Chen, S.-C. Hung, Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits , PLoS One 11 (2016) e0149835.
- H. Nejadnik, J.H. Hui, E.P. Feng Choong, B.-C. Tai, E.H. Lee, Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study , Am. J. Sports Med. 38 (2010) 1110–1116.
- I. Sekiya, T. Muneta, M. Horie, H. Koga, Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects , Clin. Orthop. Rel. Res. 473 (2015) 2316–2326.
- Y.S. Kim, Y.J. Choi, Y.G. Koh, Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes , Am. J. Sports Med. 43 (2015) 2293–2301.
- W.-L. Fu, Y.-F. Ao, X.-Y. Ke, Z.-Z. Zheng, X. Gong, D. Jiang, et al., Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment , Knee 21 (2014) 609–612.
- Y.-G. Koh, O.-R. Kwon, Y.-S. Kim, Y.-J. Choi, D.-H. Tak, Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial , Arthrosc. J. Arthrosc. Relat. Surg. 32 (2016) 97–109.
- T.S. de Windt, L.A. Vonk, I.C.M. Slaper-Cortenbach, M.P.H. van den Broek, R. Nizak, M.H.P. van Rijen, et al., Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-Stage cartilage repair in humans upon mixture with recycled autologous chondrons , Stem Cells (2016) (n/a-n/a).
- L. da Silva Meirelles, A.M. Fontes, D.T. Covas, A.I. Caplan, Mechanisms involved in the therapeutic properties of mesenchymal stem cells , Cytokine Growth Factor Rev. 20 (2009) 419–427.
- W.S. Toh, C.B. Foldager, M. Pei, J.H.P. Hui, Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration , Stem Cell Rev. Rep. 10 (2014) 686–696.
- R.C. Lai, F. Arslan, M.M. Lee, N.S.K. Sze, A. Choo, T.S. Chen, et al., Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury , Stem Cell Res. 4 (2010) 214–222.
- S. Zhang, W.C. Chu, R.C. Lai, S.K. Lim, J.H.P. Hui, W.S. Toh, Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration, Osteoarthr . Cartil. 24 (2016) 2135–2140.
- S. Zhang, W. Chu, R. Lai, J. Hui, E. Lee, S. Lim, et al., 21 – human mesenchymal stem cell-derived exosomes promote orderly cartilage regeneration in an immunocompetent rat osteochondral defect model , Cytotherapy 18 (2016) S13.
- C.T. Lim, X. Ren, M.H. Afizah, S. Tarigan-Panjaitan, Z. Yang, Y. Wu, et al., Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model
- A. Gobbi, G. Karnatzikos, S.R. Sankineani, One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee , Am. J. Sports Med. 42 (2014) 648–657.
- A. Gobbi, C. Scotti, G. Karnatzikos, A. Mudhigere, M. Castro, G.M. Peretti, One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years , Knee Surg. Sports Traumatol. Arthrosc. (2016) 1–8.
- A. Gobbi, G. Karnatzikos, C. Scotti, V. Mahajan, L. Mazzucco, B. Grigolo, One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-Year follow-up , Cartilage 2 (2011) 286–299.
- K.L. Wong, K.B.L. Lee, B.C. Tai, P. Law, E.H. Lee, J.H.P. Hui, Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up , Arthrosc. J. Arthrosc. Relat. Surg. 29 (2013) 2020–2028.
- J.M. Hare, J.E. Fishman, G. Gerstenblith, et al., Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the poseidon randomized trial, JAMA 308 (2012) 2369–2379.
- L. Wu, J.C.H. Leijten, N. Georgi, J.N. Post, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation , Tissue Eng. A 17 (2011) 1425–1436.
- L. Wu, H.-J. Prins, M.N. Helder, C.A. van Blitterswijk, M. Karperien, Trophic effects of mesenchymal stem cells in chondrocyte Co-Cultures are independent of culture conditions and cell sources , Tissue Eng. A 18 (2012) 1542–1551.
- S.K. Sze, D.P.V. de Kleijn, R.C. Lai, E. Khia Way Tan, H. Zhao, K.S. Yeo, et al., Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells , Mol. Cell. Proteomics 6 (2007) 1680–1689.
- M.B. Murphy, K. Moncivais, A.I. Caplan, Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine , Exp. Mol. Med. 45 (2013) e54.
- M.J. Lee, J. Kim, M.Y. Kim, Y.-S. Bae, S.H. Ryu, T.G. Lee, et al., Proteomic analysis of tumor necrosis factor--induced secretome of human adipose tissue-derived mesenchymal stem cells , J. Proteome Res. 9 (2010) 1754–1762.
- S. Bruno, C. Grange, M.C. Deregibus, R.A. Calogero, S. Saviozzi, F. Collino, et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury, J. Am. Soc. Nephrol. 20 (2009) 1053–1067.
- M. Yá˜nez-Mó, P.R.-M. Siljander, Z. Andreu, A.B. Zavec, F.E. Borràs, E.I. Buzas, et al. Biological properties of extracellular vesicles and their physiological functions (2015).
- C. Lawson, J.M. Vicencio, D.M. Yellon, S.M. Davidson, Microvesicles and exosomes: new players in metabolic and cardiovascular disease , J. Endocrinol. 228 (2016) R57–R71.
- A.G. Thompson, E. Gray, S.M. Heman-Ackah, I. Mager, K. Talbot, S.E. Andaloussi, et al., Extracellular vesicles in neurodegenerative diseas—pathogenesis to biomarkers, Nat. Rev. Neurol. 12 (2016) 346–357.
- I.E.M. Bank, L. Timmers, C.M. Gijsberts, Y.-N. Zhang, A. Mosterd, J.-W. Wang, et al., The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease , Expert Rev. Mol. Diagn. 15 (2015) 1577–1588.
- T. Kato, S. Miyaki, H. Ishitobi, Y. Nakamura, T. Nakasa, M.K. Lotz, et al., Exosomes from IL-1 stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes , Arthritis. Res. Ther. 16 (2014) 1–11.
- R.W.Y. Yeo, S.K. Lim, Exosomes and their therapeutic applications, in: C. Gunther, A. Hauser, R. Huss (Eds.), Advances in Pharmaceutical Cell TherapyPrinciples of Cell-Based Biopharmaceuticals, World Scientific, Singapore, 2015, pp. 477–491.
- X. Qi, J. Zhang, H. Yuan, Z. Xu, Q. Li, X. Niu, et al., Exosomes secreted by human-Induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats , Int. J. Biol. Sci. 12 (2016) 836–849.
- R.C. Lai, F. Arslan, S.S. Tan, B. Tan, A. Choo, M.M. Lee, et al., Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles , J. Mol. Cell. Cardiol. 48 (2010) 1215–1224.
- Y. Zhou, H. Xu, W. Xu, B. Wang, H. Wu, Y. Tao, et al., Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro , Stem Cell Res. Ther. 4 (2013) 1–13.
- Y. Qin, L. Wang, Z. Gao, G. Chen, C. Zhang, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo , Sci. Rep. 6 (2016) 21961.
- M. Nakano, K. Nagaishi, N. Konari, Y. Saito, T. Chikenji, Y. Mizue, et al., Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes , Sci. Rep. 6 (2016) 24805.
- K. Nagaishi, Y. Mizue, T. Chikenji, M. Otani, M. Nakano, N. Konari, et al., Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes , Sci. Rep. 6 (2016) 34842.
- S.R. Baglio, K. Rooijers, D. Koppers-Lalic, F.J. Verweij, M. Pérez Lanzón, N. Zini, et al., Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species , Stem Cell Res. Ther. 6 (2015) 1–20.
- T. Chen, R. Yeo, F. Arslan, Y. Yin, S. Tan, Efficiency of exosome production correlates inversely with the developmental maturity of MSC donor, J. Stem Cell Res. Ther. 3 (2013) 2.
- R.C. Lai, S.S. Tan, B.J. Teh, S.K. Sze, F. Arslan, D.P. de Kleijn, et al., Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome , Int. J. Proteomics 2012 (2012) 971907.
- T.S. Chen, R.C. Lai, M.M. Lee, A.B.H. Choo, C.N. Lee, S.K. Lim, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs , Nucleic Acids Res. 38 (2010) 215–224.
- R.W. Yeo, R.C. Lai, K.H. Tan, S.K. Lim, Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell, J. Circ. Biomark. 1 (2013) 7.
- R.C. Lai, R.W. Yeo, S.K. Lim, Mesenchymal stem cell exosomes, Semin. Cell Dev. Biol. 40 (2015) 82–88.
- B. Zhang, R.W. Yeo, K.H. Tan, S.K. Lim, Focus on extracellular vesicles: therapeutic potential of stem cell-derived extracellular vesicles , Int. J. Mol. Sci. 17 (2016) 174.
- Hu G-w, Q. Li, X. Niu, B. Hu, J. Liu, Zhou S-m, et al., Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice , Stem Cell Res. Ther. 6 (2015) 1–15.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li, et al., Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis , J. Transl. Med. 13 (2015) 1–14.
- B. Zhang, M. Wang, A. Gong, X. Zhang, X. Wu, Y. Zhu, et al., HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing, Stem Cells 33 (2015) 2158–2168.
- B. Zhang, Y. Yin, R.C. Lai, S.S. Tan, A.B.H. Choo, S.K. Lim, Mesenchymal stem cells secrete immunologically active exosomes , Stem Cells Dev. 23 (2013) 1233–1244.
- C.Y. Tan, R.C. Lai, W. Wong, Y.Y. Dan, S.-K. Lim, H.K. Ho, Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models , Stem Cell Res. Ther. 5 (2014) 1–14.
- C. Lee, S.A. Mitsialis, M. Aslam, S.H. Vitali, E. Vergadi, G. Konstantinou, et al., Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension , Circulation 126 (2012) 2601–2611.
- B. Yu, H. Shao, C. Su, Y. Jiang, X. Chen, L. Bai, et al., Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1 , Sci. Rep. 6 (2016) 34562.
- Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof of concept clinical trial. Stem Cells. 2014;32(5):1254–66.
- Vega, Aurelio, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
- Davatchi F, Sadeghi-Abdollahi B, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–5
- Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case- controlled study. Int Orthop. 2014;38(9):1811–1818
- Galli D, Vitale M, Vaccarezza M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:6.
- Beitzel K, Solovyova O, Cote MP, et al. The future role of mesenchymal Stem cells in The management of shoulder disorders . Arthroscopy. 2013;29(10):1702–1711.
- Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190.
- Malda, Jos, et al. " Extracellular vesicles [mdash] new tool for joint repair and regeneration. " Nature Reviews Rheumatology (2016).
Further References about PRP
- Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4(1):52–62.
Extras
- Xu, Ming, et al. " Transplanted senescent cells induce an osteoarthritis-like condition in mice. " The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (2016): glw154.
- McCulloch, Kendal, Gary J. Litherland, and Taranjit Singh Rai. " Cellular senescence in osteoarthritis pathology ." Aging Cell (2017).
Patient Services at ANOVA Institute for Regenerative Medicine
- Located in the center of Germany, quick access by car or train from anywhere in Europe
- Simple access worldwide, less than 20 minutes from Frankfurt Airport
- Individualized therapy with state-of-the-art stem cell products
- Individually planned diagnostic work-up which include world-class MRI and CT scans
- German high quality standard on safety and quality assurance
- Personal service with friendly, dedicated Patient Care Managers
- Scientific collaborations with academic institutions to assure you the latest regenerative medical programs