Are stem cell therapies a treatment alternative for Spinal Cord Injury?
Spinal Cord Injury (SCI) can be caused by physical traumata and often affects young male patients, as they show a higher risk behaviour. Although there is no possibility to date to heal SCI, several treatments exist to partially alleviate the burden of this disease, by using technology like speech control, wheel chairs and robotic arms or legs. As most patients are very young, there is a strong medial need to cure SCI completely at some point. Experimental approaches use electrical stimulation of affected areas to trigger self-healing processes but most of them are only stopping further degeneration. Exploratory clinical trials exist as well that use mesenchymal, embryonic or neural stem cells to induce and support tissue regeneration. The following chapters summarise current knowledge on SCI, standard and alternative therapies, stem cells and its secretome and how they are used as therapeutic regimen in clinical trials.
What is SCI and what are potential causes?
As the name “Spinal Cord Injury (SCI)” implies, the damage is focussed on the spinal cord which is the essential part of the central nervous system but SCI also includes damage to the vertebrae, ligaments or disks of the spinal column surrounding the spinal cord. If all sensory feeling (sensation) and motoric functions are lost below the SCI it is called “complete”, indicating that the spinal cord is more or less completely severed/damaged. If this is not the case, the SCI is defined as “incomplete”, which can be subdivided in varying degrees depending on the affected areas of your body and the functions which are lost.
The causes for the injury are in most cases physical traumata like blows to your spine which can cause dislocation, fracturing or compression of vertebrae but gunshots or knife wounds can also cut your spinal cord. The subsequent physiological reactions of the body can cause additional damage through swelling or inflammation. Arthritis, inflammation, cancer, infections or disk degeneration are examples for non-traumatic causes for SCI. Risk factors for SCI are mainly correlated with causes for injury, namely risky behaviour and alcohol abuse. Therefore, most SCI patients are male (~80% in the US) and between 16-30 years old. At the age of 65 and older the amount of SCI patients increase again as falls occur more frequently in elderly populations and diseases like osteoporosis increase as well i.
What are the current options to treat SCI?
After treating the immediate and urgent symptoms after acquiring SCI, the focus lies on preventing secondary damage that might be caused by swelling and inflammation. Ongoing care will focus on long-term problems that may arise depending on the grade of the SCI such as muscle contractures, bowel and bladder issues, respiratory infections, blood clots and deconditioning. Medications will be applied to control pain and muscular spasms and, if needed to support bladder and bowel control and sexual functioning. Unfortunately, there is no curative treatment for SCI nowadays which can restore the damage (1). Modern technologies to support SCI patients range from electronic aids (e.g. voice controlled machines) to modern wheelchairs that can climb stairs and be controlled with one hand or even the mouth. Depending on the grade of SCI there are many promising approaches using robotics to support rehabilitation or even permanent use of robotic legs/arms to alleviate the burden of SCI ii.
What are the problems with current treatment options and what needs to be changed?
As most SCI patients are rather young and the damage is (still) irreparable there is a strong medical need to investigate alternative options aside from symptomatic treatments alone. The use of robotics to allow the use of arms and legs is a great step in the right direction but cannot be applied to many people, is extremely expensive and can also cause additional side effects as the robotic arm/leg can irritate the skin over time and the patient might not even feel it. Hence, a treatment is needed that can help to regenerate SCI. It is unrealistic to hope that a completely disconnected spinal cord will be fused together in the near future but regeneration of incomplete SCI might be treated successfully with alternate treatments.
Which trustworthy alternative treatments are available to treat SCI?
Besides the current treatments to cope with symptoms and to overcome physical disabilities there are no alternative treatments available which are officially approved as being beneficial. Nevertheless, there are ongoing clinical trials and therapeutic approaches to address the medical need:
- One of the promising new approaches is intense physiotherapy training assisted by robotic devices. In contrast to the above aids, this training approach, e.g. HAL (hybrid-assisted limb) at Cyberdyne.com in Japan or Bochum Germany, is based on the idea to stimulate reinnervation of muscles in a 3 month intense training period. Since more than 1 year ANOVA co-operates with Cyberdyne and patients show great responses, even though individual results either with HAL training only or a combination of HAL and ANOVA exosome treatments. Cyberdyne is making progress in receiving insurance coverage of this robotic stimulation therapy.
- Many clinical trials focus on electrical stimulation of the affected area of the spinal cord, as electrical current is basically the “language” of our nervous system. The idea is, that the remaining nerve cells on both side of the damaged area receive stimuli and do not degenerate, with the hope that, together with other approaches, these cells might also extend/migrate towards each other and someday might lead to the partial regain of body functions (NCT04736849). ANOVA is just starting a cooperative approach with Axiobionics.com in the USA. Axiobionics produces individualized wearable electrical stimulation devices (ESD) for patients. As it is very likely that regeneration needs electrical triggers, ANOVA is currently evaluates whether the usage of wearable ESDs from Axiobionic could be a continuation of the recovery process after HAL and mesenchymal stem cell secretome (exosomes) therapy.
- While bones can regenerate and reknit, most of our other body cells are not able to multiply and repopulate injured tissue. The exception to this rule are stem cells, which are able to differentiate in certain cell types. This knowledge increased over the last decades and was applied to treat SCI by different means. There are many clinical trials ongoing, which will be described in the next section.
What kind of stem cell alternatives are offered to treat SCI?
Stem cells are well-known for their potential to differentiate and grow during our whole life, constantly replenishing various cell types. Those cells are mainly localized in our bone marrow but also in some other niches within our body. Since scientist revealed the potential of these immortal cells, they have been within focus for the development of certain technologies.
Initial experiments in rodents showed promising results for the use of mesenchymal stem cells to regenerate SCI after injection. Unfortunately, several clinical studies with this approach failed and showed no effect in human patients, even though some positive effects could be observed (less inflammation) iii. More sophisticated studies, though questionable from an ethical standpoint, use Neural Stem Cells, which showed abilities to recover motoric functions (in animals) but only when isolated from foetuses or from adult sources by invasive surgeries iv. One promising ongoing study is for example NCT04812431, which uses neural stem cells derived from embryonic stem cells to treat a specific subtype of SCI. It only started recently (end of 2021) and is estimated to be completed in September 2023.
Most of the current clinical studies focus on the use of pluripotent stem cells (PSC), either directly isolated from embryos or generated in vitro by reprogramming/inducing somatic cells into iPSCs. Depending on the source of the PSCs, the disease stage and other parameters like age and sex of the patient, the treatment in clinical trials are adjusted. The overall idea is to regenerate the damaged area but although scientist know which growth factors are needed to expand certain cell populations, they have no idea how to generate a perfect micro environment within the damaged area to achieve this goal, as all other cells within the body might react to the artificial approach of tissue regeneration. In some studies the injected iPSCs are genetically modified or pre-stimulated to overexpress certain growth factors (still pre-clinical in animals). Ongoing clinical studies use PSCs in vitro, differentiate them in different cell types which might be favourable (Oligodendrocytes, NCT02302157, NCT01217008) and inject them at the site of injury. Results for study NCT02302157 showed that every patient had at least one adverse event but none suffered severe health damage. Unfortunately, the results were rather weak, showing only minor benefits on motoric functions of the upper extremities, more or less independent of the treatment regimen (dosage, frequency).
n short: injection of stem cells to effectively treat SCI is still in its infancy. Nevertheless, it could be shown that injection of stem cells does indeed reduce inflammatory reactions even though it does not regenerate the nerve damage itself. On this basis, ANOVA uses its stem cell-based secretome therapy in a combinatory approach (see below).
How does ANOVA treat SCI
ANOVA IRMs treatment basis is the hypothesis, that after incomplete spinal cord injury, the spinal cord has the potential to partially recover but this primarily needs sufficient muscle stimulation by any type of physiotherapy or electrical stimulation. In the second line, the suppression of inflammation and thereby a change in the regenerative potential is synergistic if not essential. In contrast to other approaches, ANOVA aims at using the proven anti-inflammatory potential of stem cells as one part in a complex combinatory approach. As a consequence, ANOVA only treats SCI patients with incomplete injury and remaining detectable signals.
As a consequence, ANOVA strongly recommends high impact physiotherapy, best with HAL training, and views the stem cell treatment as the supportive and permissive combinatory treatment. After HAL training physiotherapy should be continued. As the ANOVA secretome is produced in 10 doses, the complete time of HAL training is paralleled by secretome treatment.
What are potential risks of treating SCI with stem cells?
Stem cell isolation, their cultivation and in vitro expansion are quite complex procedures, especially if they are meant to be reinjected in vivo. On the one hand, the risk of contamination and subsequent rejection of the cells might occur. On the other hand, cells that are expanded too rapidly might loose their potency or might transform into cancer cells if treated with complex cocktails of growth factors for too long. Hence, the whole process must be strictly controlled throughout the whole life cycle of the cells, from isolation to reinjection or the therapy might cause adverse effects or be simply ineffective. Patients should always ask for legal permits if they consider treatment as this ensures quality and safety.
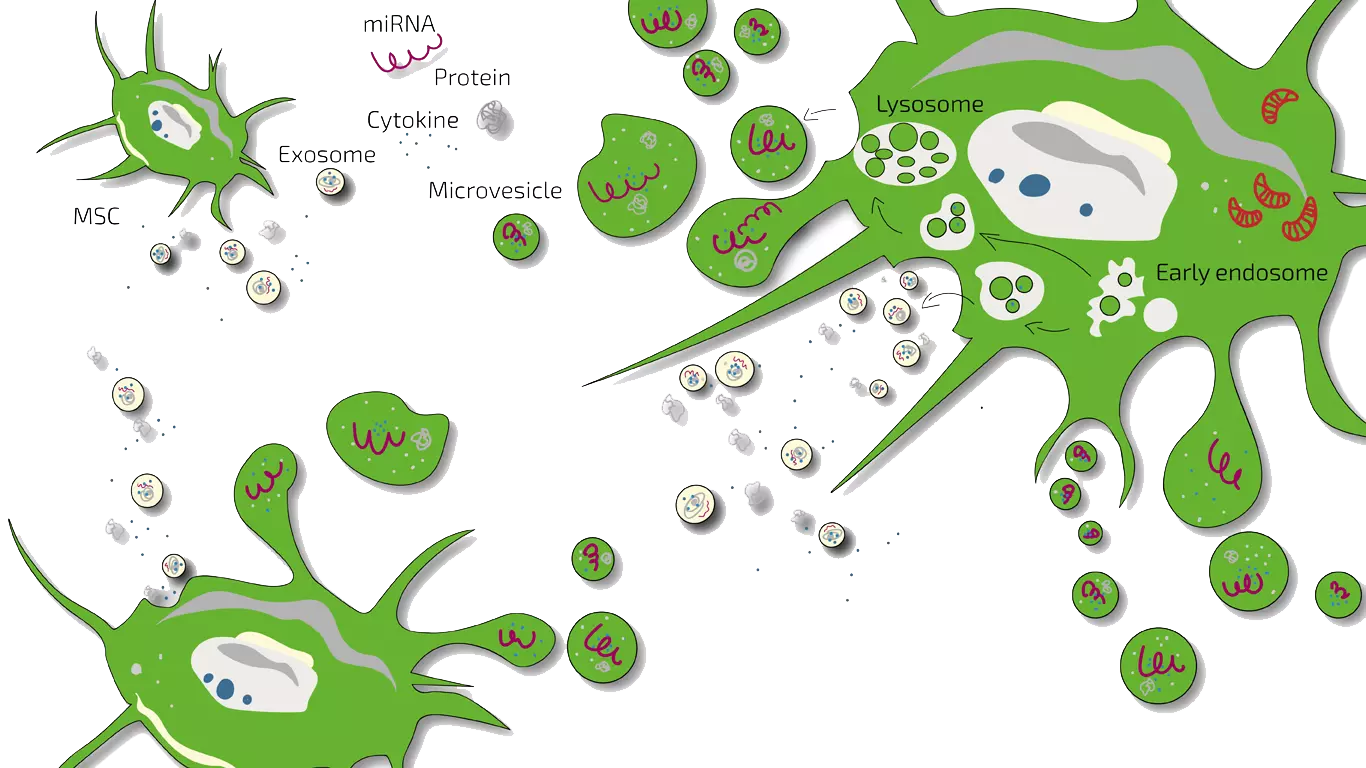
What is the stem cell secretome?
One reason for the observed beneficial effects after injection of stem cells are believed to be the secreted compounds (exosomes, cytokines or other biologically active proteins) into their so-called “microenvironment”. The entirety of these secreted compounds, which are partially incorporated within exosomes as well as microvesicles and partially are suspended in the liquid phase, is called “secretome”. Scientists and clinicians may use the complete secretome but in most cases use only the isolated exosomes which lack the soluble factors.
Several publications already showed that this secretome contains neuroprotective growth factors which can protect neurons and glial cells and at the same time activate anti-inflammatory and anti-apoptotic pathways within the surrounding tissue v, vi. It is also discussed if exosomes transfer miRNA which play a key role in inter-cellular communication vi. Scientific data supports the theory that injection of stem cell conditioned media can mitigate the severity of the injury and enhance functional recovery of spinal cord injury in animal models vi. Results from animal models for ALS showed that the use of stem cells or its secretome does have beneficial effects but it appears as if the secretome alone is sufficient, indicating that it is probably not necessary to inject the stem cells.
What is the ANOVA IRM secretome?
The secretome provided by ANOVA IRM contains exosomes and soluble secreted factors and is fully legally controlled. It does not contain cells that carry the risk of malignancy as it is proven cell-free. ANOVA IRM was the first institution world-wide to acquire legal permission by the regulatory bodies for this type of product in 2018.
Only one donation is necessary to isolate your own personal, so-called autologous, mesenchymal stem cells from belly fat which are then cultivated in vitro for a short period to generate sufficient amounts of minimally-manipulated stem cell-conditioned medium which contains the exosomes and soluble factors secreted by your stem cells. After separating the stem cells from the medium, your own personalized secretome will undergo a thorough pharmaceutical quality control before it is being used for up to 10 treatments, which will be scheduled according to your special needs, usually in parallel to the 3 months HAL training period. All internal processes are controlled by German Drug Authorities and are therefore highly regulated to yield proven quality and highest safety available.
What are the advantages?
There are many advantages when using the ANOVA secretome over the injection of stem cells. As the stem cells are cultivated over a short period of time and under mild conditions there is only a low risk of loss of potency during the cultivation. No cells have to be reinjected and therefore the risk for infections or the risk to develop cancer from the pluripotent stem cells does not exist. Your own personalized secretome-containing medium will be generated and stored until you need it. The freezing does not largely change the composition. In contrast, classical MSC treatments are 1 time therapies which does not seem reasonable, as stem cells are not a cure and therefore, on-going treatment is needed. If stem cells are frozen, their behavior seems to change rendering them potentially less effective which does not allow for freezing for repeated therapy. Finally, you can rely on the highly regulated system of the German Drug Authorities when it comes to quality and safety of the final product.
Whether Stem Cell Secretome is a treatment option for you depends on your current health status and the underlying problem in your specific case. If you would like to know more about your treatment options with Stem Cell Secretome, schedule an appointment today. Our scientists and doctors will assess your medical files to see if you can benefit from stem cell treatment.
News & Insights
References and Literature
- MajoClinic. (2022), vol. https://www.mayoclinic.org/diseases-conditions/spinal-cord-injury/symptoms-causes/syc-20377890.
- M. Mekki, A. D. Delgado, A. Fry, D. Putrino, V. Huang, Robotic Rehabilitation and Spinal Cord Injury: a Narrative Review. Neurotherapeutics 15, 604-617 (2018).
- F. Cofano et al., Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int J Mol Sci 20, (2019).
- B. Fernandez-Munoz, A. B. Garcia-Delgado, B. Arribas-Arribas, R. Sanchez-Pernaute, Human Neural Stem Cells for Cell-Based Medicinal Products. Cells 10, (2021).
- S. Marconi et al., Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A 18, 1264-1272 (2012).
- S. Shin et al., Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton's Jelly. Int J Mol Sci 22, (2021).
Contraindications
Our stem cell treatments are experimental, but we only treat patients for whom we believe the risk/benefit ratio indicates treatment based on the state of the art, i.e., medical, scientific evidence.
Please understand that we therefore do not treat patients for whom the following points apply:
- Active cancer in the last two years
- Not yet of legal age
- Existing pregnancy or lactation period
- Unable to breathe on own, ventilator
- Difficulty breathing in supine position
- Dysphagia (extreme difficulty swallowing)
- Psychiatric disorder
- Active infectious disease (Hepatitis A, B, C, HIV, Syphilis, or other)
Patient Services at ANOVA Institute for Regenerative Medicine
- Located in the center of Germany, quick access by car or train from anywhere in Europe
- Simple access worldwide, less than 20 minutes from Frankfurt Airport
- Individualized therapy with state-of-the-art stem cell products
- Individually planned diagnostic work-up which include world-class MRI and CT scans
- German high quality standard on safety and quality assurance
- Personal service with friendly, dedicated Patient Care Managers
- Scientific collaborations with academic institutions to assure you the latest regenerative medical programs